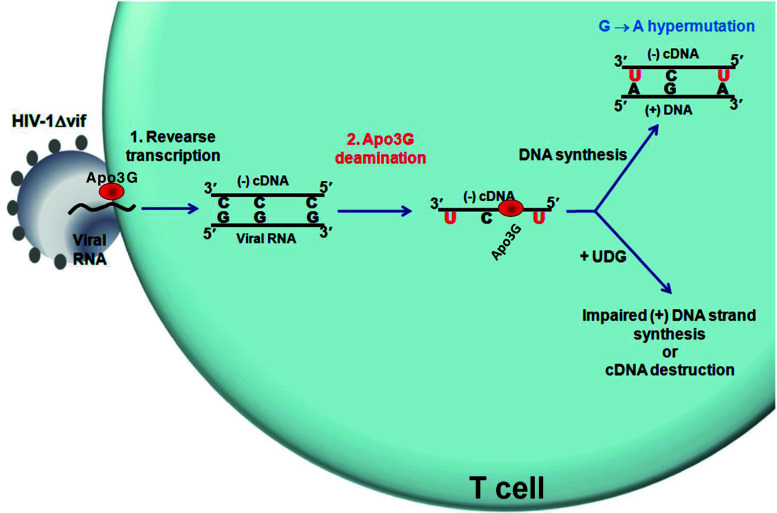
Fig. 2.
Apo3G-dependent restriction of HIV-1. Apo3G is encapsidated into Vif-deficient (∆vif) HIV-1 virions. Upon infection, Apo3G enters the cytoplasm of the targeted T cell and deaminates multiple C on the HIV-1 reverse transcribed minus (−) cDNA strand creating a massive number of U residues. Synthesis of the (+) strand, using U as templates, introduces hypermutation in essential genes, effectively inactivating HIV-1 infectivity. Alternatively, abasic sites resulted from the removal of U by the cellular enzyme, uracil DNA glycosylase, could inhibit the synthesis of the HIV-1 (+) strand cDNA or could serve as substrates for apurinic-apyrimidinic endonucleases leading to degradation of the cDNA reverse transcribed intermediate