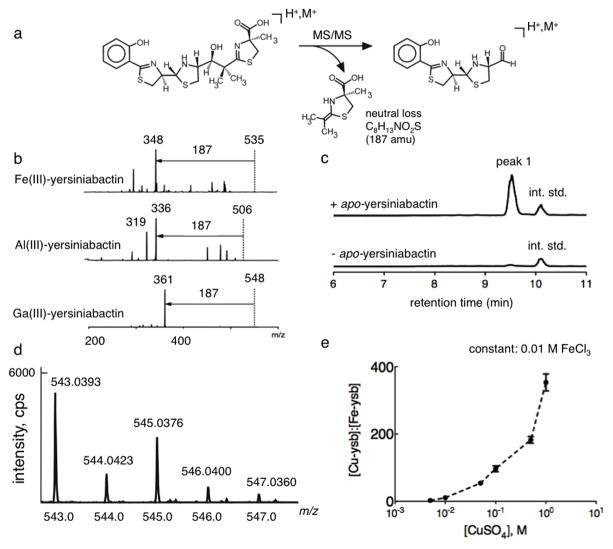
Figure 1. A yersiniabactin neutral loss screen reveals formation of a novel copper(II)-yersiniabactin complex in human urine.
(a) Yersiniabactin and its metal complexes exhibit a dominant 187 mass unit neutral loss upon CID fragmentation of the positive ESI-derived ion. This neutral loss is consistent with rearrangement to lose the terminal carboxylated thiazoline. (b) A 187 mass unit neutral loss is evident in MS/MS product ion spectra of ferric-yersiniabactin (Fe(III)-Ybt) at m/z 535, aluminum-yersiniabactin (Al(III)-Ybt) at m/z 506, and gallium-yersiniabactin (Ga(III)-Ybt) at m/z 548. A constant neutral loss (CNL) scan based on this conserved fragmentation pathway was used as a metallomic screen to identify physiologic yersiniabactin binding partners. (c) Representative constant neutral loss chromatograms of urine samples in the presence and absence of purified apo-yersiniabactin. The combination of apo-yersiniabactin and urine results in formation of a prominent new peak (peak 1). Peaks corresponding to the internal standard (int. std) are indicated. These results were confirmed in three independent experiments. (d) High resolution positive ion ESI mass spectrum is consistent with the empiric formula for a singly charged Cu(II)-Ybt ion and demonstrates the prominent natural abundance M+2 ion expected from 65Cu. (d) Competitive binding experiments were conducted by titrating cupric sulfate into solutions containing a fixed concentration of 0.01 M ferric chloride and 0.01 M apo-yersiniabactin. Data indicate competitive binding between cupric and ferric ion for the ligand. Cu(II)-Ybt/Fe(III)-Ybt ratios were determined by comparing selected ion chromatogram peak ratios to those from a standard curve.