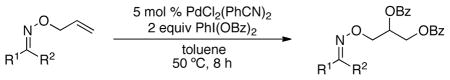
Table 2.
Alkene Dioxygenation with Chiral Auxiliaries Derived from Commercial Ketonesa
| |||
|---|---|---|---|
| substrate | product | yieldb | drc |
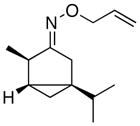 (E-8) |
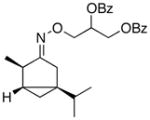 (E-8a) |
64% | 63:37 |
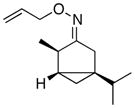 (Z-8) |
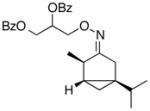 (Z-8a) |
63% | 52:48 |
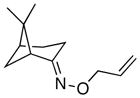 (9) |
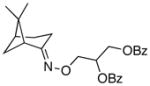 (9a) |
68% | 59:41 |
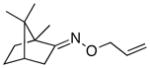 (E-10) |
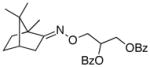 (E-10a) |
25% | 75:25 |
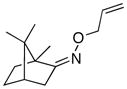 (Z-10) |
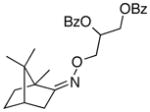 (Z-10a) |
67% | 66:34 |
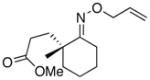 (11) |
 (11a) |
<1%d | -- |
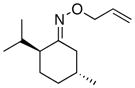 (12) |
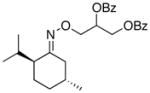 (12a) |
75% | 74:26 |
Conditions: substrate (1 equiv), PdCl2(PhCN)2 (0.05 equiv), PhI(OBz)2 (2 equiv), dry toluene (0.12 M in substrate), 50 °C, 8 h.
Isolated yield.
Determined by relative integrations of at least 2 pairs of peaks in the 13C and/or 1H NMR spectra; see Supporting Information for details.
Product was not detected by NMR spectroscopic analysis of the crude reaction mixture.
