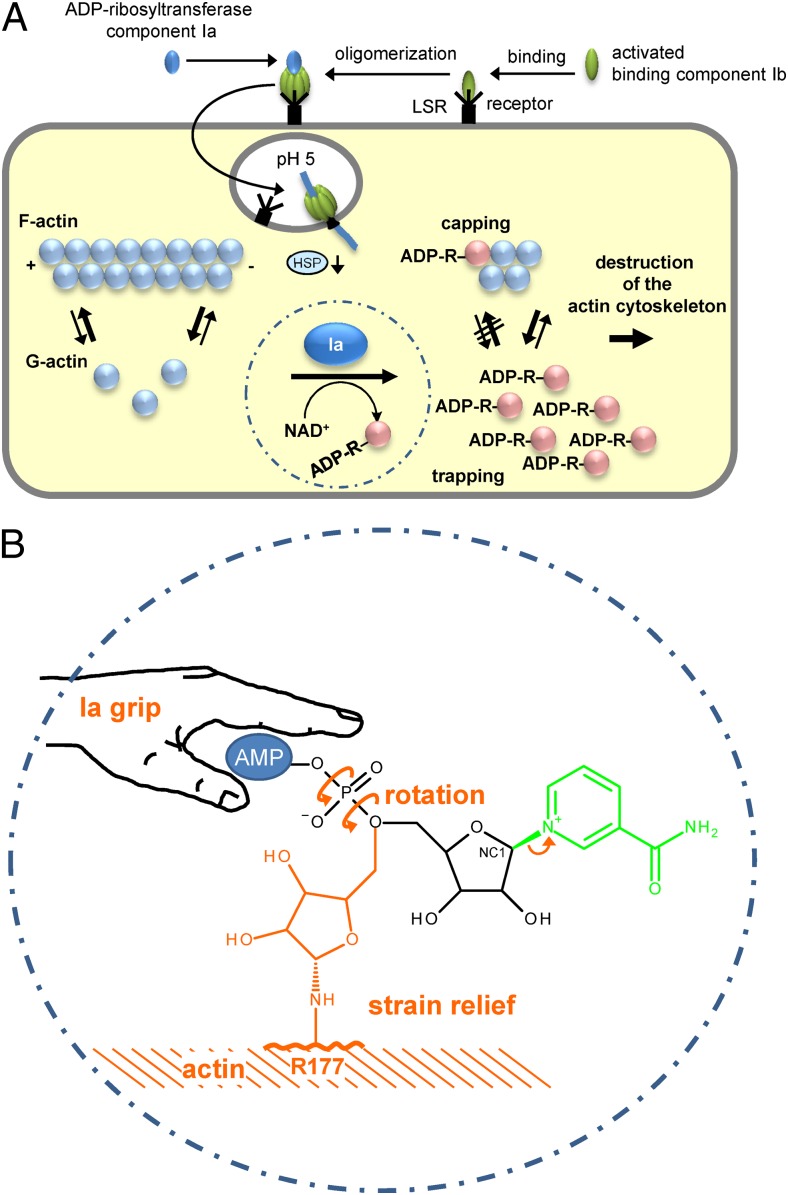
Fig. 1.
(A) Up-take and action of C. perfringens iota toxin. Iota toxin consists of the ADP-ribosyltransferase component Ia and the receptor binding component Ib. Both are activated by proteolytic cleavage (not shown). Ib binds to its cell surface receptor LSR (lipolysis-stimulated lipoprotein receptor) (5) and forms heptamers to which Ia binds. The toxin-LSR complex is endocytosed. At low pH of endosomes the toxin heptamers insert into the vesicle membrane and translocate Ia into the cytosol with the help of cellular chaperons (e.g., heat-shock protein 90, HSP). In the cytosol, Ia ADP-ribosylates monomeric G-actin in Arg177. ADP-ribosylated actin is not able to polymerize and is trapped in the monomeric form (trapping). Moreover, ADP-ribosylated actin acts as a capping protein to block polymerization of nonmodified actin. (B) Mechanism of ADP ribosylation of actin by Ia suggested by Tsurumura et al. (3). The ADP ribosyltransferase component Ia binds the AMP moiety of NAD+ with a tight grip in a prereaction state. ADP ribosylation reaction starts with release of nicotinamide and formation of a first oxocarbenium cation intermediate (small arrow). Thereafter, a rotation around the axes of the phosphodiester bond forms a second oxocarbenium cation, which results in strain relief and brings the C1 (NC1) of the ribosyl moiety near to arginine177 (R177) of actin to complete the transfer of ADP ribose.