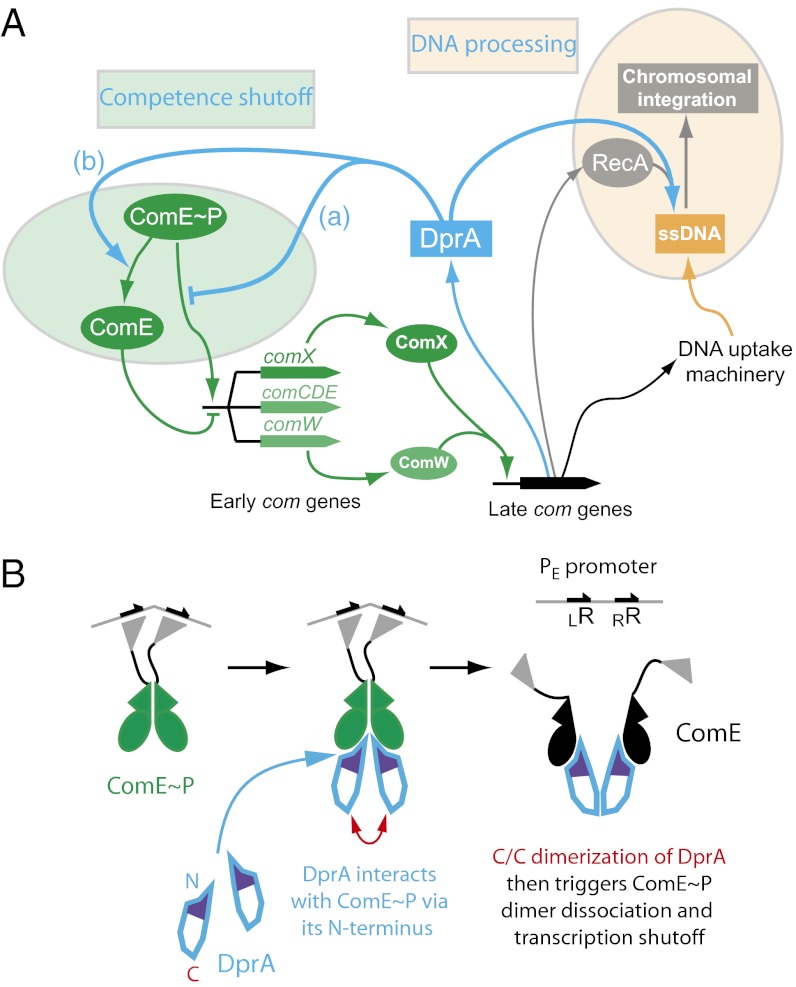
Fig. 6.
A dual role for DprA: RecA loading and competence shut-off. (A) DprA was first recognized as playing a key role in the processing of internalized ssDNA as a transformation-dedicated RecA loader. The present study documents its direct involvement in the shut-off of competence. We propose that DprA similarly affects PcomX and PcomW, controlling, respectively, expression of the competence-specific σX factor and ComW, which is crucial for σX activation and stabilization. DprA could act as an antiactivator (a) or affect ComE phophorylation state (b) (Discussion). (B) Diagrammatic representation of the involvement of DprA C/C dimerization in the shutoff of PE promoters. DprA could first interact with ComE∼P as a monomer, through its N terminus. C/C dimerization of two monomers could then trigger ComE∼P dimer dissociation. As RR phosphorylation mediates dimerization, which then promotes transcriptional activation (12), ComE∼P dimer dissociation is likely to result in transcription shut-off. Possible alternatives are evoked in the Discussion.