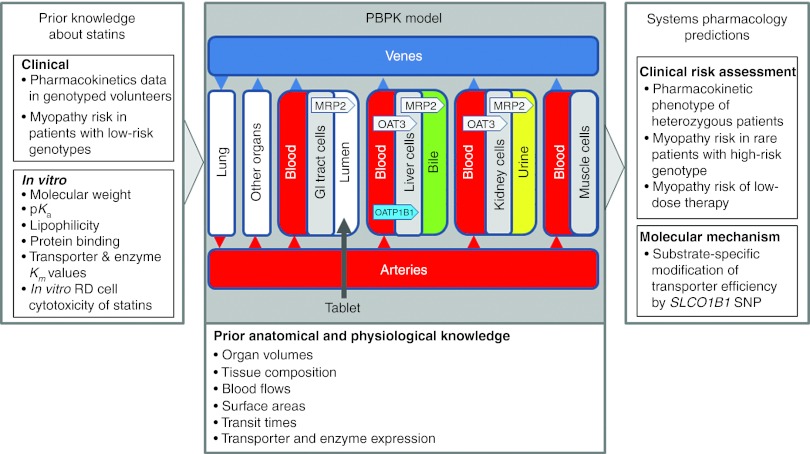
Figure 1.
Systems pharmacology. A generic systems pharmacology approach exploits drug safety–related information to predict adverse event rates. Physiology-based pharmacokinetic models are used to represent large amounts of prior physiological, anatomical, and pharmacological information and knowledge. The workflow additionally integrates preclinical data and clinical trial results routinely generated drug development. Together with the existing knowledge of genetic risk factors, the approach quantifies potential safety issues in high-risk patient populations. GI, gastro-intestinal; RD, embryonal rhabdomyosarcoma cells; SNP, single-nucleotide polymorphism.