Abstract
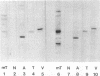
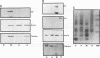
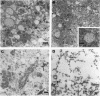
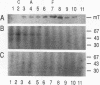
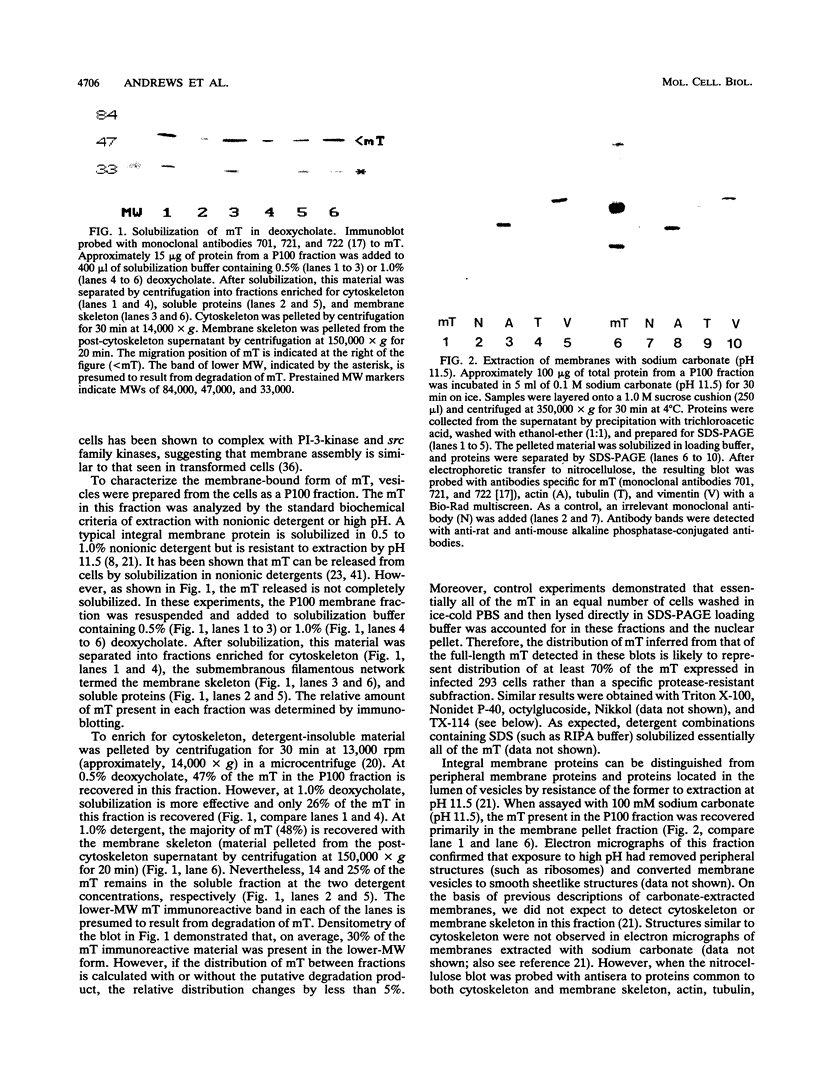
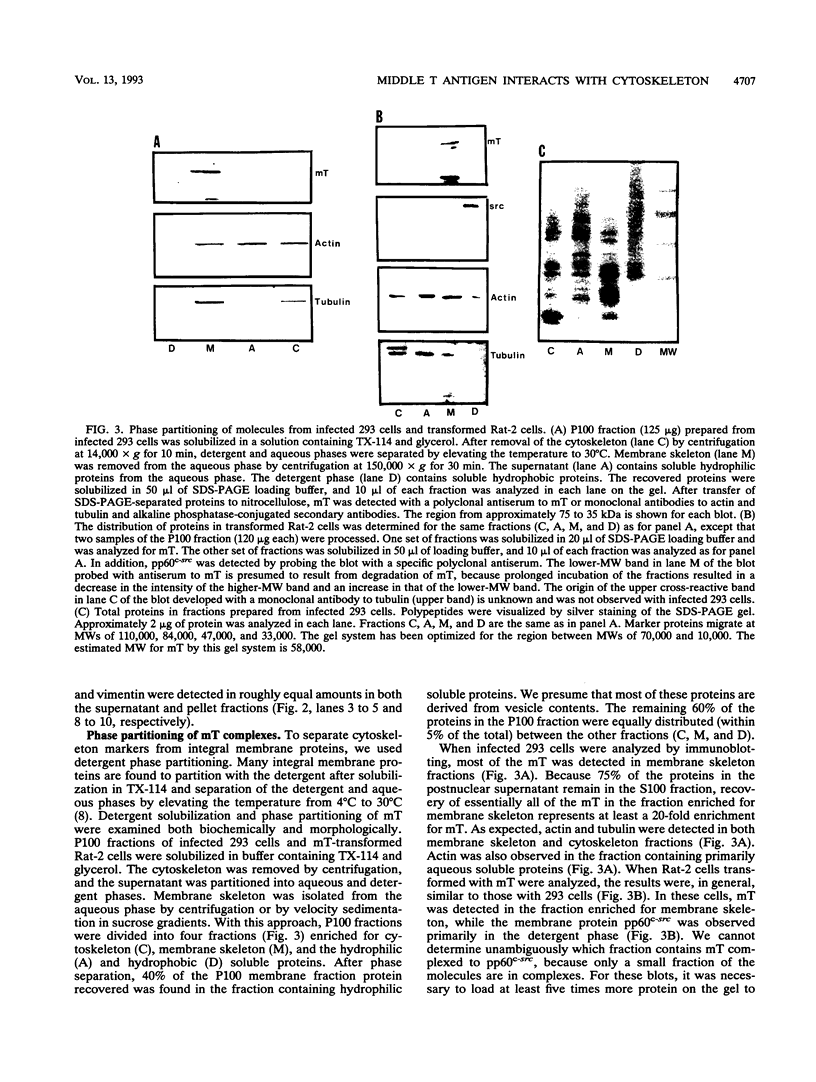
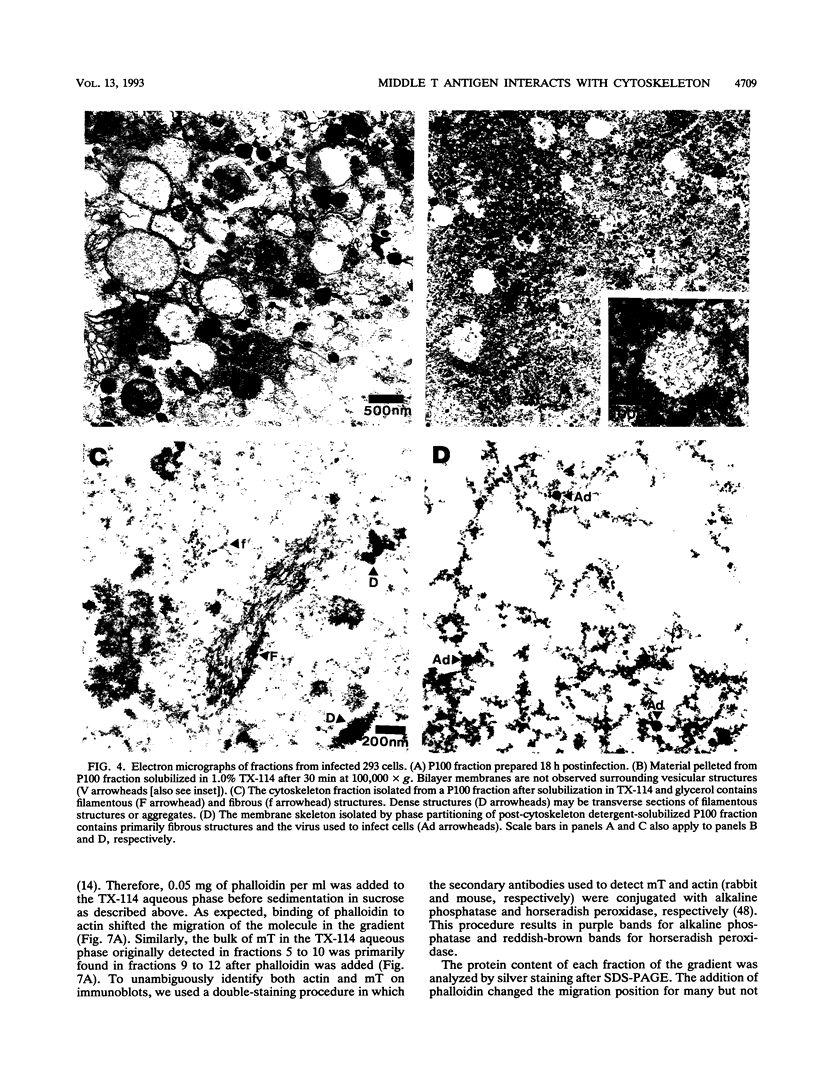
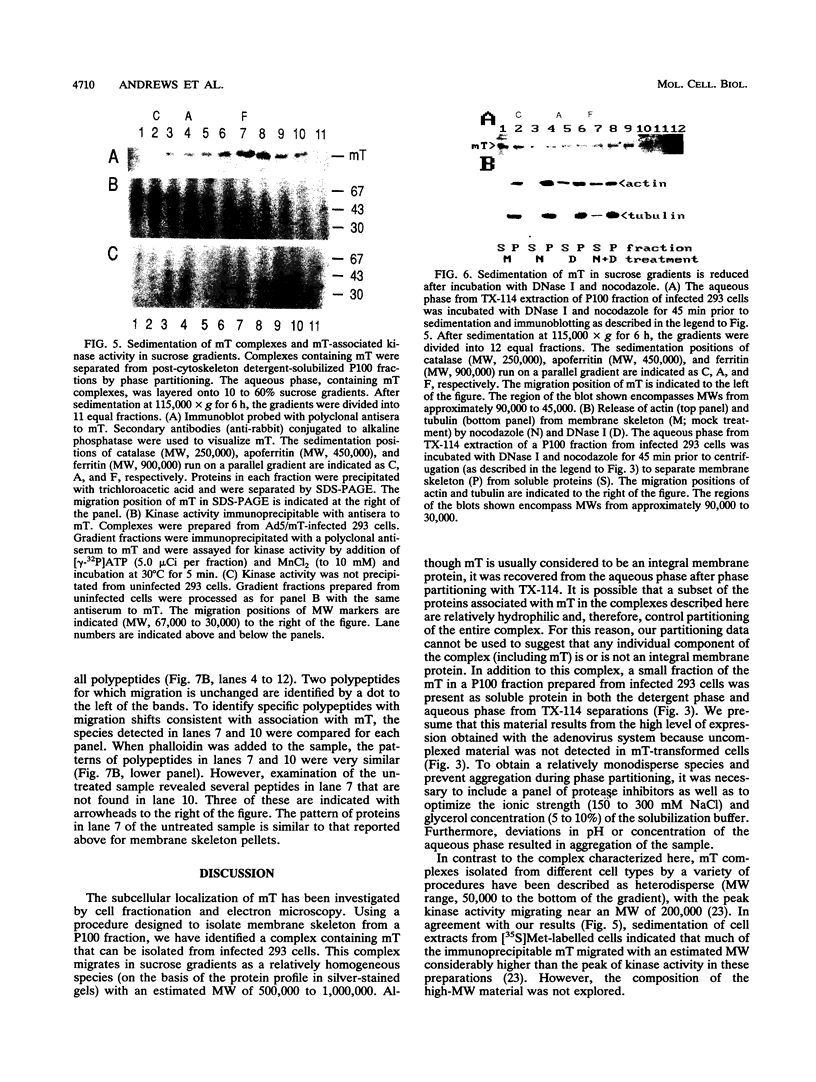
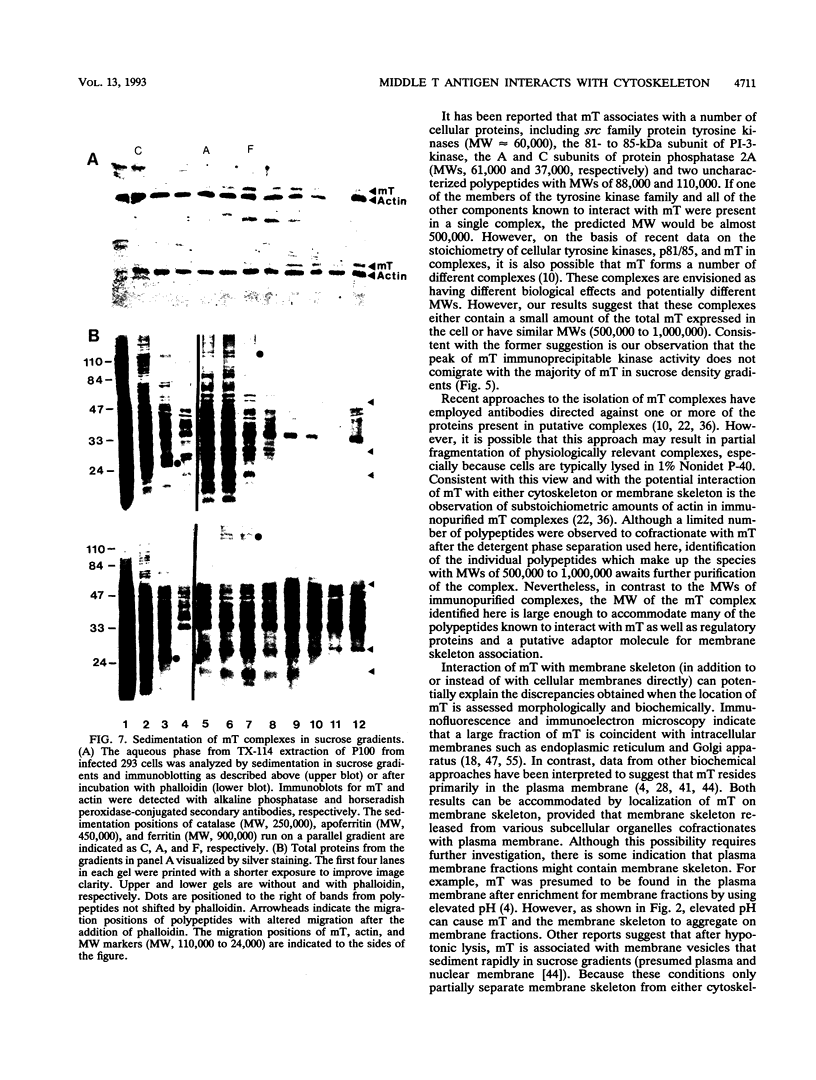
The transforming protein of polyomavirus, middle T antigen, is associated with cellular membranes. We have examined the subcellular location of the middle T antigen in two different cell types by fractionation and detergent phase partitioning. Middle T antigen expressed in human cells by a recombinant adenovirus was detected primarily in the membrane skeleton. Sucrose gradient fractionation revealed that the middle T antigen was associated with complexes with molecular weights of 500,000 to 1,000,000. Several markers for cytoskeleton cofractionate with these complexes, including actin, tubulin, and vimentin. Electron micrographs of membrane skeleton prepared from cells expressing middle T antigen demonstrated that this material contained primarily fibrous structures and was clearly devoid of bilayer membranes. These structures were distinct from the filamentous structures observed in fractions enriched for cytoskeleton. Consistent with a role for membrane skeleton localization in transformation, middle T antigen was detected exclusively in fractions enriched for membrane skeleton in middle T antigen-transformed Rat-2 cells. Our results may resolve the apparent difference between middle T antigen localization as determined by immunomicroscopy and that determined by subcellular fractionation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguzzi A., Kleihues P., Heckl K., Wiestler O. D. Cell type-specific tumor induction in neural transplants by retrovirus-mediated oncogene transfer. Oncogene. 1991 Jan;6(1):113–118. [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Polyomavirus middle T antigen downregulates junctional cell-to-cell communication. Mol Cell Biol. 1987 Feb;7(2):946–950. doi: 10.1128/mcb.7.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Ballmer-Hofer K., Benjamin T. L. Phosphorylation of polyoma middle T antigen and cellular proteins in purified plasma membranes of polyoma virus-infected cells. EMBO J. 1985 Sep;4(9):2321–2327. doi: 10.1002/j.1460-2075.1985.tb03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Bendzko P., Prehn S., Pfeil W., Rapoport T. A. Different modes of membrane interactions of the signal sequence of carp preproinsulin and of the insertion sequence of rabbit cytochrome b5. Eur J Biochem. 1982 Mar;123(1):121–126. doi: 10.1111/j.1432-1033.1982.tb06507.x. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Espino P. C., Marshall J., Harvey R., Smith A. E. Stoichiometry of cellular and viral components in the polyomavirus middle-T antigen-tyrosine kinase complex. Mol Cell Biol. 1990 Oct;10(10):5569–5574. doi: 10.1128/mcb.10.10.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Harvey R., Espino P. C., Semba K., Yamamoto T., Toyoshima K., Smith A. E. Peptide antibodies to the human c-fyn gene product demonstrate pp59c-fyn is capable of complex formation with the middle-T antigen of polyomavirus. EMBO J. 1988 Dec 1;7(12):3845–3855. doi: 10.1002/j.1460-2075.1988.tb03270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Liu Y. X., Druker B., Roberts T. M., Schaffhausen B. S. Characterization of pp85, a target of oncogenes and growth factor receptors. Mol Cell Biol. 1990 Jun;10(6):2909–2915. doi: 10.1128/mcb.10.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. N., Pavloff N., Hassell J. A. Simultaneous overexpression of avian pp60c-src and polyomavirus middle T antigen in mammalian cells. J Virol. 1990 May;64(5):2392–2395. doi: 10.1128/jvi.64.5.2392-2395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Strittmatter P. Structural and functional properties of the membrane binding segment of cytochrome b5. J Biol Chem. 1978 Nov 25;253(22):8203–8209. [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Hansson H. A., Darnfors C., Bjursell G., Streuli C. H., Griffin B. E. Subcellular localisation of the middle and large T-antigens of polyoma virus. EMBO J. 1986 Mar;5(3):491–499. doi: 10.1002/j.1460-2075.1986.tb04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Boyles J. K., Berndt M. C., Steffen P. K., Anderson L. K. Identification of a membrane skeleton in platelets. J Cell Biol. 1988 May;106(5):1525–1538. doi: 10.1083/jcb.106.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grussenmeyer T., Carbone-Wiley A., Scheidtmann K. H., Walter G. Interactions between polyomavirus medium T antigen and three cellular proteins of 88, 61, and 37 kilodaltons. J Virol. 1987 Dec;61(12):3902–3909. doi: 10.1128/jvi.61.12.3902-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grussenmeyer T., Scheidtmann K. H., Hutchinson M. A., Eckhart W., Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Hanafusa H. Localization of major potential substrates of p60v-src kinase in the plasma membrane matrix fraction. Oncogene Res. 1989;4(1):29–37. [PubMed] [Google Scholar]
- Horak I. D., Kawakami T., Gregory F., Robbins K. C., Bolen J. B. Association of p60fyn with middle tumor antigen in murine polyomavirus-transformed rat cells. J Virol. 1989 May;63(5):2343–2347. doi: 10.1128/jvi.63.5.2343-2347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A. R., Muszbek L., Kellie S. Translocation of pp60c-src to the cytoskeleton during platelet aggregation. EMBO J. 1992 Mar;11(3):855–861. doi: 10.1002/j.1460-2075.1992.tb05123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Brocklehurst J. R., Dulbecco R. Virus-specific proteins in the plasma membrane of cells lytically infected or transformed by pol-oma virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4666–4670. doi: 10.1073/pnas.74.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Cheng S. H., Markland W., Fukui Y., Hanafusa H. Association of p62c-yes with polyomavirus middle T-antigen mutants correlates with transforming ability. J Virol. 1990 Apr;64(4):1584–1589. doi: 10.1128/jvi.64.4.1584-1589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., Sudol M., Hanafusa H. Association of the polyomavirus middle-T antigen with c-yes protein. Nature. 1987 Jan 8;325(7000):171–173. doi: 10.1038/325171a0. [DOI] [PubMed] [Google Scholar]
- Liebl E. C., Martin G. S. Intracellular targeting of pp60src expression: localization of v-src to adhesion plaques is sufficient to transform chicken embryo fibroblasts. Oncogene. 1992 Dec;7(12):2417–2428. [PubMed] [Google Scholar]
- Lin K. H., Cheng S. Y. An efficient method to purify active eukaryotic proteins from the inclusion bodies in Escherichia coli. Biotechniques. 1991 Dec;11(6):748, 750, 752-3. [PubMed] [Google Scholar]
- Ling L. E., Druker B. J., Cantley L. C., Roberts T. M. Transformation-defective mutants of polyomavirus middle T antigen associate with phosphatidylinositol 3-kinase (PI 3-kinase) but are unable to maintain wild-type levels of PI 3-kinase products in intact cells. J Virol. 1992 Mar;66(3):1702–1708. doi: 10.1128/jvi.66.3.1702-1708.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Cheng S. H., Oostra B. A., Smith A. E. In vitro mutagenesis of the putative membrane-binding domain of polyomavirus middle-T antigen. J Virol. 1986 Jul;59(1):82–89. doi: 10.1128/jvi.59.1.82-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markland W., Smith A. E. Mutants of polyomavirus middle-T antigen. Biochim Biophys Acta. 1987 Nov 25;907(3):299–321. doi: 10.1016/0304-419x(87)90011-4. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Cherington V., Morgan W., DeAnda J., Kaplan D., Schaffhausen B., Roberts T. M. Cellular proteins that associate with the middle and small T antigens of polyomavirus. J Virol. 1988 Nov;62(11):3934–3940. doi: 10.1128/jvi.62.11.3934-3940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Morgan W., Roberts T. M. The cellular proteins which can associate specifically with polyomavirus middle T antigen in human 293 cells include the major human 70-kilodalton heat shock proteins. J Virol. 1989 Nov;63(11):4533–4539. doi: 10.1128/jvi.63.11.4533-4539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Williams N. G., Cheng S. H., Roberts T. M. Regulation of pp60c-src and its interaction with polyomavirus middle T antigen in insect cells. J Virol. 1990 Jan;64(1):61–68. doi: 10.1128/jvi.64.1.61-68.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Courtneidge S. A., Loubière R., el Baze P., Cuzin F. A variety of tumours induced by the middle T antigen of polyoma virus in a transgenic mouse family. Oncogene. 1990 Oct;5(10):1507–1510. [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Dorai H., Arakere G., Benjamin T. L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982 Oct;2(10):1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serunian L. A., Auger K. R., Roberts T. M., Cantley L. C. Production of novel polyphosphoinositides in vivo is linked to cell transformation by polyomavirus middle T antigen. J Virol. 1990 Oct;64(10):4718–4725. doi: 10.1128/jvi.64.10.4718-4725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D., Voronova A., Eckhart W. Construction and expression of a recombinant DNA gene encoding a polyomavirus middle-size tumor antigen with the carboxyl terminus of the vesicular stomatitis virus glycoprotein G. Mol Cell Biol. 1984 Feb;4(2):282–289. doi: 10.1128/mcb.4.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen N., Lohoff E. S., von Figura K., Hasilik A. Sequential detection of antigens in Western blots with differently colored products. Anal Biochem. 1986 Feb 1;152(2):211–214. doi: 10.1016/0003-2697(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Ulug E. T., Cartwright A. J., Courtneidge S. A. Characterization of the interaction of polyomavirus middle T antigen with type 2A protein phosphatase. J Virol. 1992 Mar;66(3):1458–1467. doi: 10.1128/jvi.66.3.1458-1467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Bautch V. L. The polyomavirus early region gene in transgenic mice causes vascular and bone tumors. J Virol. 1991 Oct;65(10):5174–5183. doi: 10.1128/jvi.65.10.5174-5183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Risau W., Zerwes H. G., Drexler H., Aguzzi A., Wagner E. F. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell. 1989 Jun 16;57(6):1053–1063. doi: 10.1016/0092-8674(89)90343-7. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]