Abstract
Background
Spasticity after stroke has been internationally recognized as an important health problem causing impairment of mobility, deformity, and pain. The aim of this study was to assess the frequency of first-ever and recurrent stroke and of subsequent spastic and flaccid paresis. Factors influencing the development of spasticity were analyzed. A further major aim was to provide a “real-life” assessment of the treatment of spasticity in Germany and to discuss this in view of the treatment recommended by German and international clinical guidelines.
Methods
The database used in this study comprised a cohort of 242,090 insurants from a large statutory health insurance fund in the federal state of Hesse, Germany. A first hospital discharge diagnosis in 2009 with any of the International Classification of Diseases, Tenth Revision (ICD-10) codes I60–I64 was used to identify patients with acute stroke (hemorrhage and ischemic). These patients were followed up six months after stroke to monitor whether they developed spastic or flaccid paresis (hospital or ambulatory care diagnoses ICD-10 code G81–G83 [excluding G82.6/G83.4/G83.8]). For patients with spastic paresis after stroke the spasticity treatment was analyzed for a six-month period (physiotherapy, oral muscle relaxants, intrathecal baclofen, and botulinum toxin).
Results
Standardized to the population of Germany, 3.7 per 1000 persons suffered a stroke in 2009 (raw 5.2/1000). Of all surviving patients, 10.2% developed spasticity within 6 months. Cox regression revealed no significant influence of patient age, gender, morbidity (diabetes, hypertensive diseases, ischemic heart diseases) or type of stroke on development of spasticity. 97% of surviving patients with spasticity received physiotherapy (inpatient care 89%, ambulatory care 48%). Oral muscle relaxants were prescribed to 13% of the patients. No patient received intrathecal baclofen or botulinum toxin.
Conclusion
Claims data enabled analysis of the occurrence of stroke and post-stroke spasticity. These data provide insight into real-life treatment for spasticity in Germany. The proportion of patients who receive physiotherapy, which is the international guideline-recommended basic therapy after transition into ambulatory care, can be improved on. Botulinum toxin as an international guideline-based treatment option for focal spasticity has not been implemented in practice in Germany as yet.
Keywords: health care utilization, physiotherapy, drug therapy, claims data
Introduction
Stroke and post-stroke sequelae, including paresis and spasticity, are regarded as important health problems worldwide.1,2 Depending on the severity of spasticity, impairment of mobility, deformity, and pain can be the result. As a consequence, the patients’ ability to perform self-care tasks is often substantially impaired and the provision of adequate professional care is a major challenge, eg, because the flexion deformity of the limbs may interfere with skin care, dressing, and seating. Compared with stroke, data on the frequency of post-stroke spasticity are sparse. Depending on setting and methodology, the published studies report a wide range of point prevalences of 18% to 42% at distinct examination time points within the first year after stroke.2–9 For any patient with spasticity, international clinical guidelines for the management of stroke recommend simple procedures to reduce spasticity, like exercise and stretching. For patients with persistent moderate to severe spasticity (ie, interfering with activities or care), botulinum toxin in combination with rehabilitation therapy is internationally recommended.10–15 The guidelines differ in their appraisal of other therapies, such as antispastic medication or intrathecal baclofen. In 2008, the German Society of Neurology evaluated medical and nonmedical treatment options for spasticity as part of their guidelines for diagnostics and therapy.16 The guideline for treatment of spasticity recommends physiotherapy as basic therapy for all patients. Additional drug treatment with oral muscle relaxants, local botulinum toxin injection, or intrathecal baclofen, might facilitate movements in affected joints. The choice of drug therapy depends on the medical condition of individual patients and should be determined on a case-by-case basis.
Some studies are available for the German population on frequency of stroke17–19 and development of spasticity after stroke.7 However, apart from a Thai register study9 and a Swedish cost of illness study20 that present some data on health care utilization, we could not identify any further studies that have compared the treatment of post-stroke spasticity in routine health care with actual treatment recommendations. Therefore, as a first step, we determined the frequency of acute (first-ever and recurrent) stroke and the incidence of subsequent spastic paresis in our sample. We further investigated the influence of patient age, gender, previous morbidity of diabetes, hypertensive, or ischemic heart diseases and type of stroke on the development of spastic paresis. The main objective of our study was to investigate the type of treatment that patients with post-stroke spasticity receive in routine health care and to discuss the utilization of this treatment in view of German and international clinical guideline recommendations.10–16
Materials and methods
Health care system
For a better understanding of the study and the results presented, a brief description of the German health care system would be helpful. About 90% of the German population are insured by statutory health insurance, with a free choice of health insurance fund (to date, about 150 different funds exist). In contrast with many other health care systems, the ambulatory and hospital sector are separated. In ambulatory care, general practitioners and specialists work single-handed in solo or group practices. Physiotherapists can work in their own practice or be employed by a specialist (mostly an orthopedic specialist). Patients with stroke are mostly referred to a hospital, but are monitored and treated after discharge by general practitioners or specialists (eg, neurologists) in the ambulatory care setting. Neurologists and psychiatrists cannot be distinguished in claims data, because they are coded as one profession. Physicians in ambulatory care have a certain drug budget for the insured population. In the event that they exceed the budget they run the risk of being subrogated. Drug prices in hospitals are mostly cheaper compared with prices in ambulatory care. In general, in the ambulatory care setting, patients have a free choice of physician. For the first visit to a physician and for each physician visited without referral, a copayment of 10 Euros per quarter had to be made at that time by all but chronically ill patients. Furthermore, copayments are required for each prescribed drug and for a prescription of physiotherapy. For remuneration purposes, all care providers have to deliver data concerning patients, services, and treatments provided, and the diagnoses made to the health insurance funds. Since 2000, physicians in ambulatory care have to code the diseases of their patients according to International Classification of Diseases, Tenth Revision, German Modification21 (ICD-10-GM) with the following modifier: assured, suspicion, excluded, status after. The coded diagnoses are submitted to the sickness fund. They are not linked to a specific consultation with the patient but only to the corresponding quarter of the year. If a disease persists for a longer period, the diagnosis has to be documented in each quarter in which the respective disease was treated. Hospital diagnoses are coded without any modifier. Admission diagnosis, discharge diagnosis, and secondary diagnosis are provided and coded according to ICD-10-GM code. For the time being, there is no coding option available to express whether a certain condition has been “present on admission”.
Database
The study was based on routine health care data of a large German statutory health insurance fund (AOK, Die Gesundheitskasse, a German health insurance fund) located in the federal state of Hesse. Nationwide, the AOK covers approximately 37% of all members of the statutory health insurance system in Germany, corresponding to slightly less than a third of the total German population of 82 million people. It consists of 14 regional funds, of which one covers the federal state of Hesse situated in west-central Germany, with 6 million inhabitants. Health insurance provided data for an 18.75% random sample of all insured persons for this study.22 All data were pseudonymized and include information on patient age and gender, period of insurance cover, and inpatient and ambulatory care received. The study was conducted according to the German Social Security Code X, §75 “Transfer of social data for research and planning” and approved by the Hessian Ministry of Social Affairs. Ethical approval was not required in view of the nonexperimental design of the study, and in line with the German good practice guideline for analysis of routine data.23 The source population contains 242,090 people, all continuously insured between 2008 and 2010, and includes persons who died in 2009 and 2010.
Study population with acute stroke
Patients who suffered from acute stroke due to hemorrhage or cerebral infarction in 2009 were identified by their first hospital discharge diagnosis of the year with one of the ICD-10-GM codes,21 ie, I60–I64. Validation studies carried out in Finland, England and the USA showed a good validity of ICD hospital discharge diagnosis of stroke.24–26 No German validation study has been conducted on this topic. However, we consider the hospital discharge coding in Germany to be valid as the diagnoses are relevant for remuneration purposes and therefore are controlled. The date of hospital admission was deemed to be the date of the stroke event. All cases of acute stroke (first-ever and recurrent) were included as the type of stroke has no influence on the treatment of the post-stroke spasticity. The prevalence of acute stroke was calculated per 1000 and standardized according to the age and sex distribution of the population of Germany on 31.12.2009. 95% Clopper-Pearson confidence intervals (CI) were computed for proportions. The 95% confidence intervals for the standardized stroke rates were calculated by non-parametric Bootstrap (using the bias-corrected and accelerated method).27
Patients with a diagnosis of paresis up to 180 days (inpatient care) or two quarters (ambulatory care) before stroke were excluded from the subsequent analysis. Hence, patients with acute stroke in 2009 (first-ever or recurrent) without diagnosis of paresis in the 6 months before their stroke were included into the subsequent analysis.
Study population with post-stroke spasticity/paresis
Patients were followed up for six months after their stroke (observation period 1) for inpatient or ambulatory diagnoses of paresis: ICD-10-GM codes G81 (hemiplegia), G82 (paraplegia and tetraplegia), and G83 (other paralytic syndromes, excluding G82.6, G83.4, G83.8). Patients who received a G81.1, G82.1, or G82.4 ICD-10-GM diagnosis (spastic hemiplegia, paraplegia, or tetraplegia, respectively) in hospital or ambulatory care were assumed to suffer from spastic paresis. We included all diagnoses of paresis marked as “assured” and coded in the quarter of the stroke or in one of the two following quarters. The first documentation of spasticity, ie, hospital admission date or the date of the first visit to the documenting physician in ambulatory care, served as the date of onset of spasticity. The percentage of newly diagnosed paresis patients in the stroke population was computed along with 95% Clopper-Pearson confidence intervals. The numbers are given separately for survivors and deceased to reveal possible differences. Figure 1 gives an overview of the numbers of observed patients, definition of cases, and observation periods.
Figure 1.
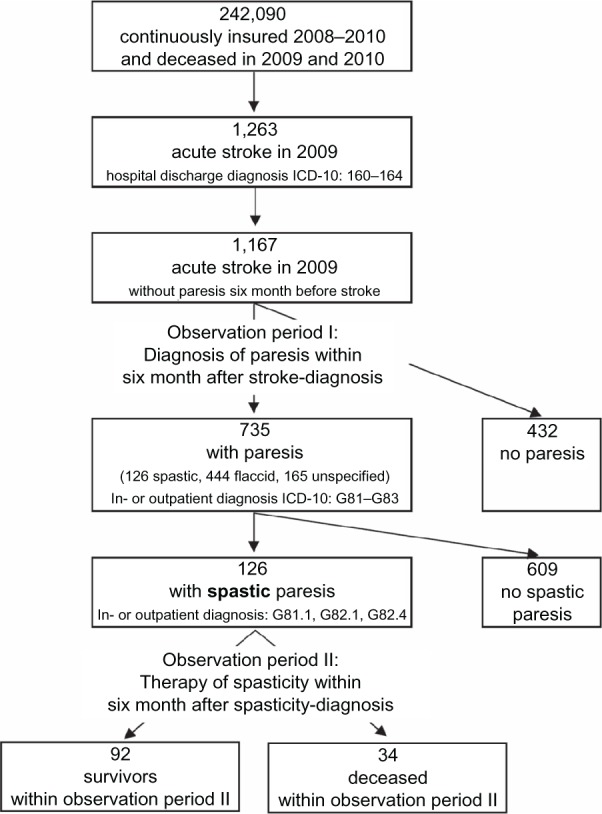
Numbers of observed patients, definition of cases, and observation periods.
Factors influencing the development of spasticity
We used Cox regression to analyze the influence of age, gender, previous morbidity and type of stroke on the development of spastic paresis compared with no paresis. Previous morbidity was determined as an assured diagnosis of diabetes mellitus (ICD-10-GM codes E10–E14), hypertensive disease (I10–I15), or ischemic heart disease (I20–I25) within six months before stroke. Patients with a diagnosis of unspecified or flaccid paresis were excluded from the analysis. Thus, 558 stroke patients with no paresis or spastic paresis were included in our analysis. The level of significance was set at α = 0.05. All statistical analyses were performed using the SAS software version 9.2 (SAS Institute, Cary, NC, USA).
Analysis of spasticity treatment
The German clinical guideline on therapy of spasticity recommends physiotherapy as basic treatment for all patients, and additional drug therapy in individual cases, depending on severity.16 We determined the percentage of spastic patients who received nonmedical treatment or drug therapy within six months after the first diagnosis of spasticity (observation period 2) and computed corresponding 95% Clopper-Pearson confidence intervals. In the data the treatment is not linked to a specific diagnosis. Furthermore, the analyzed treatments are indicated in other diseases as well. Therefore, we analyzed changes in the frequency of the treatment of interest in the spasticity population by comparing the six-month period before stroke with the six-month period after diagnosis of spasticity. Data are presented separately for survivors and deceased cases to reveal possible differences.
To analyze nonmedical therapy, we considered the following services: inpatient physiotherapy also with complex inpatient neurological therapy, early-onset rehabilitation therapy, and inpatient rehabilitation and physiotherapy in ambulatory care. The services are reimbursed according to the documented codes (operation procedure classification codes for inpatients, diagnosis-related groups for patients in ambulatory care; German doctors’ fee scale,28 AOK-specific codes). The code numbers can be made available from the authors upon request.
For analysis of drug therapy using oral muscle relaxants, we included drugs according to the Anatomical Therapeutic Chemical classification system (ATC) with ATC codes M03B (muscle relaxants, centrally acting agents), M03CA01 (dantrolene), as well as the benzodiazepines clonazepam (N03AE01) and diazepam (N05BA01). To cover intrathecal baclofen therapy, we included prescriptions of baclofen in ampullae (M03BX01) for ambulatory care and operation procedure classification code 8011, for the intrathecal application of drugs (inpatient therapy). Botulinum toxin injections were assessed by including prescriptions with ATC codes M03AX or operation procedure classification code 6003.8 (application of drugs, botulinum toxin).
Results
Frequency of acute stroke
In 2009, 1263 of the 242,090 insurants (sample population) were admitted to a hospital for acute stroke (first-ever or recurrent). The mean age of the stroke patients was 75 ± 11.9 (range 5–100) years. For men (48%, 604 in total) the mean age was 71 ± 11.2 (range 9–94) years and for women (52%, 659 in total) was 78 ± 11.8 (range 5–100) years. Stroke occurred at a rate of 5.2 per 1000 (95% CI 4.9–5.5). Standardized to the German population (reference date, December 31, 2009) by age and gender, the rate was 3.7 per 1000 (95% CI 3.4–3.9). Table 1 shows the rates stratified by type of stroke and gender.
Table 1.
Cases of acute stroke in 2009
| Stroke ICD-10 classification | Total n | Total per 1000 (95%-CI) | Men per 1000 (95%-CI) | Women per 1000 (95%-CI) |
|---|---|---|---|---|
| Hemorrhage (I60–I62) | 167 | 0.7 (0.6–0.8) | 0.7 (0.5–0.8) | 0.7 (0.6–0.9) |
| Cerebral infarction (I63) | 1054 | 4.4 (4.1–4.6) | 4.4 (4.1–4.8) | 4.3 (3.9–4.6) |
| Stroke, not specified (I64) | 42 | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.2 (0.1–0.3) |
| Total | ||||
| Raw | 1263 | 5.2 (4.9–5.5) | 5.3 (4.8–5.7) | 5.2 (4.8–5.6) |
| Standardized | 3.7 (3.4–3.9) | 3.8 (3.5–4.1) | 3.5 (3.2–3.8) | |
Notes: Source population n = 242,090, standardized to the population of Germany (reference date December 31, 2009).
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; CI, confidence interval.
Spastic paresis after acute stroke
Of 1263 patients with acute stroke, 96 were excluded from the analysis due to diagnosis of paresis up to six months prior to acute stroke. In total, we observed 1167 patients for six months after stroke with regard to documentation of a diagnosis of paresis (observation period 1). Results for patients who died during this period are presented separately; 59.6% of survivors and 75.1% of deceased patients developed paresis (see Table 2 providing details according to type of diagnosis). Within six months after stroke a diagnosis of spasticity was documented in 10.2% of survivors. Even though the observation period was shorter for non-survivors as the event of death occurred during the study period, the percentage of non-survivors developing spasticity was slightly higher (12.8%). However, corresponding confidence intervals overlap, ie, differences were non-significant. Patients with flaccid paresis may develop spastic paresis later on. We found that both, flaccid and spastic paresis, were documented within the 6-month observation period for 4.3% of all survivors and 5.8% of all deceased stroke patients.
Table 2.
Paresis within six months of acute stroke
| Within six months of acute stroke (observation period 1)
|
||||
|---|---|---|---|---|
| Survivors (n = 910)
|
Deaths (n = 257)
|
|||
| n | % (95% CI) | n | % (95% CI) | |
| No paresis | 368 | 40.4 (37.2–43.7) | 64 | 24.9 (19.7–30.7) |
| Spastic paresis (G81.1, G82.1, G82.4) | 93 | 10.2 (8.3–12.4) | 33 | 12.8 (9.0–17.6) |
| Flaccid paresis (G81.0, G82.0, G82.3) | 312 | 34.3 (31.2–37.5) | 132 | 51.4 (45.1–57.6) |
| Unspecified paresis# | 137 | 15.1 (12.8–17.6) | 28 | 10.9 (7.4–15.4) |
| ICD-10 diagnoses | ||||
| G81 Hemiplegia | 489 | 53.7 (50.4–57.0) | 186 | 72.4 (66.5–77.8) |
| G82 Paraplegia and tetraplegia | 8 | 0.9 (0.4–1.7) | 5 | 1.9 (0.6–4.5) |
| G83 Other paralytic syndromes | 45 | 5.0 (3.6–6.6) | 2 | 0.8 (0.1–2.8) |
Notes: n = 1167 patients with stroke and no diagnosis of paresis up to six months before stroke.
ICD-10: G81.2, G82.2, G82.5, G83 without G83.4, G83.8.
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; CI, confidence interval.
Factors influencing development of spasticity
Table 3 shows the factors, age, gender, type of stroke and previous morbidity analyzed using Cox regression to determine their influence on the development of spastic paresis as compared with no paresis. None of these factors had a significant influence on the development of spasticity.
Table 3.
Factors analyzed for influence on development of spasticity with Cox regression
| Characteristics | Spasticity
|
|||
|---|---|---|---|---|
| yes | no | HR | 95% HR CI | |
| Patients (%) | 126 (22.6) | 432 (77.4) | ||
| Age, mean ± SD, years | 73 ± 11.8 | 74 ± 12.1 | 1.00 (per yearly increment) | 0.98–1.02 |
| Men (%) | 64 (50.8) | 212 (49.1) | Ref | |
| Women (%) | 62 (49.2) | 220 (50.9) | 1.03 | 0.72–1.47 |
| Cerebral infarction (I63) (%) | 109 (86.5) | 354 (81.9) | Ref | |
| Hemorrhage (I60–I62) (%) | 14 (11.1) | 61 (14.1) | 0.78 | 0.44–1.39 |
| Stroke, not specified (I64) (%) | 3 (2.4) | 17 (4.0) | 0.62 | 0.20–1.94 |
| Diabetes mellitus (E10–E14)*(%) yes vs no | 48 (38.1) | 142 (32.9) | 1.24 | 0.84–1.80 |
| Hypertensive diseases (I10–I15)*(%) yes vs no | 84 (66.7) | 312 (72.2) | 0.77 | 0.51–1.18 |
| Ischemic heart diseases (I20–I25)*(%) yes vs no | 38 (30.2) | 129 (29.9) | 1.05 | 0.70–1.58 |
Notes: n = 558 patients with spastic paresis versus with no paresis (patients with only unspecified or flaccid diagnosis of paresis were excluded).
Assured diagnosis within six month prior to stroke. HR adjusted for variables listed in the table are presented.
Abbreviations: HR, hazard ratio; CI, confidence interval; SD, standard deviation.
Treatment of spasticity
Treatment for spasticity was analyzed based on 126 patients who developed spastic paresis in the six months following their stroke. Guideline-recommended therapies, ie, physiotherapy and muscle relaxant drug therapy were performed during the six months after the initial diagnosis of spasticity (observation period 2). The proportion of post-stroke spasticity patients receiving treatments such as physiotherapy increased considerably in the six-month period after onset of spasticity. The proportion of patients receiving inpatient physiotherapy was comparable between survivors and nonsurvivors (approximately 88%). Of all the survivors, 47.8% received physiotherapy in ambulatory care compared with 8.8% of nonsurvivors (Table 4). Of the non-survivors who contacted an ambulatory care physician following their hospital stay, ie, who had the chance to receive a prescription of physiotherapy, 12% underwent physiotherapy in ambulatory care.
Table 4.
Patients with spasticity: physiotherapy and muscle-relaxing drug therapy before stroke and after diagnosis of spasticity
| Therapy | Period of therapy,
|
|||||
|---|---|---|---|---|---|---|
| Six month before stroke n = 126
|
Six month after onset of spasticity (observation period 2)
|
|||||
| n | % (95% CI) | Survivors n = 92
|
Deaths n = 34
|
|||
| n | % (95% CI) | n | % (95% CI) | |||
| Physiotherapy | ||||||
| Inpatient | 10 | 7.9 (3.9–14.1) | 82 | 89.1 (80.9–94.7) | 30 | 88.2 (72.6–96.7) |
| Outpatient | 14 | 11.1 (6.2–17.9) | 44 | 47.8 (37.3–58.5) | 3 | 8.8 (1.9–23.7) |
| Total physiotherapy | 21 | 16.7 (10.6–24.3) | 89 | 96.7 (90.8–99.3) | 30 | 88.2 (72.6–96.7) |
| Oral drug therapy | ||||||
| Baclofen | 1 | 0.8 (0.02–4.3) | 9 | 9.8 (4.6–17.8) | 1 | 2.9 (0.1–15.3) |
| Tizanidine | 0 | – | 1 | 1.1 (0.03–5.1) | 0 | – |
| Others | 4 | 3.2 (0.9–7.6) | 4 | 4.3 (1.2–10.8) | 1 | 2.9 (0.1–15.3) |
| Total | 5 | 4.0 (1.3–9.0) | 12 | 13.0 (6.9–21.7) | 2 | 5.9 (0.7–19.7) |
| Baclofen intrathecal | 0 | – | 0 | – | 0 | – |
| Botulinum toxin injection | 0 | – | 0 | – | 0 | – |
Abbreviation: CI, confidence interval.
One patient (0.8%) received oral baclofen therapy before diagnosis of stroke versus 9.8% of the surviving patients after onset of spasticity. Other muscle relaxants already prescribed before stroke were diazepam (n = 1), tetrazepam (n = 2), and tolperisone (n = 1). After onset of spasticity, tinzanidin (n = 1), diazepam (n = 4), methocarbamol (n = 1), and tolperisone (n = 1) were prescribed. Overall, 4% of patients received oral muscle relaxants in the six months before their stroke, whereas utilization of this therapy increased to 13% after development of spasticity. No patient received intrathecal baclofen therapy or botulinum toxin (Table 4).
Discussion
Acute stroke
Standardized to the German population by age and gender, 3.7 per 1000 insured persons suffered from acute stroke (first-ever and recurrent) in 2009 according to our data. Compared with other studies in Germany,18,19 we determined a higher rate of acute stroke because we did not restrict our analysis to first-ever stroke. For example, when analyzing claims data for 24.1 million AOK insurants, Günster et al reported a standardized first-ever stroke incidence rate of 2.7 per 1000 for the year 2008, excluding 25% of patients with stroke or transient ischemic attack within a 5-year period prior to stroke (ICD-10, I60–I64; age- and gender-adjusted to the German population in 2007).18 A study by van den Bussche et al based on 1,700,000 insurants of the German statutory health insurer, Gmünder Ersatzkasse, found a much lower hospitalized first-ever stroke incidence rate of 1.23 per 1000 in 2006 (ICD-10, I60–I64, gender-adjusted).19 In addition to methodological differences, which hamper the comparison of rates, one has to take into account that there are hints that insurees in the different sickness funds might have different morbidity risks, even when adjusting for gender and age.29
Spastic paresis after acute stroke
Several international and national clinical studies have followed up first-time stroke patients for spasticity (point prevalence at examination time) using the modified Ashworth Scale for clinical assessment.2 The observed prevalences for spasticity vary substantially across studies. A Swedish study by Lundström et al found a rate of spasticity of 18% among 140 patients one year after stroke (mean age of participants 71 ± 13 years; 48% women).4 An earlier Swedish study by Sommerfeld et al reported a 21% rate of spasticity a few days after stroke and a rate of 19% 3 months after stroke (95 patients; mean age 78 ± 9.5 years; 63% women).5 Further follow-up of the patients found a rate of spasticity of 20% among 66 patients still under observation after 18 months (mean age 76 ± 10 years; 67% women).6 In contrast, a German study by Wissel et al7 and a UK-based study by Watkins et al reported higher prevalences;8 Wissel et al found the following rates of spasticity (initial cohort 103 patients, mean age 69 [range 35–96] years; 38% women): 24.5% of 94 patients at baseline (median 6 days after stroke), 26.7% of 86 patients at first follow-up (median duration until disease onset 6 weeks after stroke), and 21.7% of 83 patients at second follow-up (median 16 weeks after stroke).7 The study by Watkins et al, which included patients with previous stroke, found that 27% of 106 patients had developed spasticity during the 12 months following stroke (mean age 70 ± 11 years; 49% women).8 They found no significant differences in rates of spasticity between first-ever stroke patients and patients with previous stroke. Again, the setting is relevant when comparing rates. The Thai register study, for example, reported a high prevalence of post-stroke spasticity of 41.6% in 327 enrolled patients, but could not rule out that this high rate was due to potentially nonrepresentative selection of patients entering inpatient rehabilitation after development of spasticity.9
In the study by Welmer et al, spasticity was significantly more common in younger stroke patients 3 months, but not 18 months, after stroke diagnosis.30 Lundström et al presented similar findings based on patients with severe and disabling forms of spasticity.4 Their results indicate that the influence of age may be significant only for a certain period of time and in specific subgroups with severe spasticity.
Our analysis showed that age, gender, type of stroke, and previous morbidity did not significantly influence the development of spasticity and hence do not explain the different findings of the modified Ashworth Scale-based studies. Further, we found a much lower rate of spasticity of 10.2% among survivors within 6 months after stroke (period prevalence). Possible explanations are that low modified Ashworth grades are not routinely or are insufficiently documented in routine patient care. In addition, the low rate might be explained by the fact that about 15% of the patients received an unspecified diagnosis that did not classify the paresis as flaccid or spastic. We found an increased prescription of oral muscle relaxants also among patients with an unspecified diagnosis, albeit lower than among patients with spasticity diagnosis. This indicates that patients with spasticity are among patients with an unspecified diagnosis. Therefore, the “real” percentage of spastic paresis may be higher.
Treatment of spasticity
We analyzed the implementation of options chosen to treat spasticity along with corresponding frequencies in comparison with clinical guideline recommendations from the German Society of Neurology16 and the German consensus statement on botulinum toxin in the treatment of adult spasticity, an interdisciplinary German 10-point consensus from 2010.31 In accordance with international guidelines,10–15 the German guideline recommends physiotherapy as basic treatment for all patients with spastic paresis. One sixth of the patients analyzed had already received physiotherapy six months before their stroke. This finding is not surprising because physiotherapy is indicated for many disorders, including back pain and fractures. However, during the six months following the onset of spasticity, the percentage of patients receiving physiotherapy increased considerably to 97% among survivors and 88% among patients who died within six months after the onset of spasticity. Given that physiotherapy may to some extent be prescribed for other post-stroke sequelae, we may have overestimated the use of physiotherapy for spasticity. Compared with the percentage of inpatients receiving physiotherapy, the percentage of survivors treated after transition into ambulatory care is low (89% versus 48%). We can only speculate about the reasons for this, such as limited possibilities for patients to reach available services, lack of information, or inability to perform physiotherapy due to deteriorating health status. Wissel et al32 recently addressed barriers preventing seamless post-stroke care for different countries. They argue for case managers and discharge planning to improve post-acute ambulatory care. In the US Behavioral Risk Factor Surveillance System survey, only about 31% of stroke survivors received post-hospital rehabilitation.33 That research stresses the importance of family and caregiver support for ensuring receipt and continuation of outpatient rehabilitation. Physiotherapy is a strongly recommended basic treatment for spasticity. In the light of our findings, its utilization should be increased.
In addition to physiotherapy, the German guideline recommends oral muscle relaxants for immobile patients with severe spasticity to reduce muscle tone, to improve the passive range of movement and, subsequently, to facilitate care and hygiene. However, the guideline points out that functional improvement, such as active movement, cannot be expected after treatment with oral muscle relaxants. Baclofen and tizanidine are considered to be the drugs of choice because of their small number of side effects. International guidelines10–13,15 only restrictedly recommend oral agents, eg, for generalized spasticity10,11 or in case of painful, disabling spasticity15 and refer to the side effects of the drugs.13,15 In our study, the frequency of treatment with muscle relaxants, especially baclofen, did increase within six months after onset of spasticity compared with the six month before stroke. Not unexpectedly, we found prescriptions of oral muscle relaxants before stroke because they are also used for the treatment of other conditions, such as spinal disorders and muscle pain. The scope of the data does not allow us to assess the appropriateness of oral muscle relaxant prescribing in individual spasticity cases due to lack of information on disease severity.
According to international and German clinical guidelines, intrathecal baclofen should be restricted to patients suffering from severe, chronic spastic paraparesis and tetraparesis who cannot be successfully treated with physiotherapy and standard drug therapy.10–12,15,16,34 Spastic paraparesis/tetraparesis is a rare complication after stroke, and not surprisingly, we found only two patients with this diagnosis in our data set of 1167 stroke patients. Spastic hemiplegia is a further, albeit less important, indication for intrathecal baclofen therapy because studies have shown a positive effect, especially on associated pain. Given that intrathecal baclofen is rarely indicated, it is not surprising that no patient in our study received this therapy.
The German clinical guideline and the consensus statement16,30 as well as international guidelines10–15,35 classify local botulinum toxin injection as evidence-based therapy for focal and regional spasticity. In contrast with other European countries, botulinum toxin is only licensed in Germany for the treatment of spasticity of the upper and not the lower extremities. The authors of the consensus paper recommend a realistic individual target, such as reduction of pain, ease of care, prevention of secondary complications, and improvement of function or self-perception.30 They estimate that botulinum toxin therapy is indicated in at least 5000 additional patients with spasticity after stroke annually in Germany.30 However, no patient in our database received such treatment. In order to check whether botulinum toxin injections are sufficiently covered by our data, we compared the total amount of defined daily doses of botulinum toxin prescribed for any indication to all insurants in our database with the published number of defined daily doses prescribed to all insurants of statutory health insurances in Germany.36 Extrapolation of our data to the number of all insurants in Germany revealed that the amount of prescribed defined daily doses are comparable between our study sample and all German insurants.
Botulinum toxin is not implemented as treatment for spasticity in Germany so far. Studies on barriers to guideline implementation point out general implementation barriers, eg, lack of guideline awareness or agreement, as well as setting specific barriers.37 The authors of the UK national guideline of stroke explicitly mention implications of their treatment recommendations of post-stroke spasticity that might explain barriers of implementation, ie, need for provision of a specialized service for patients with severe spasticity in every geographical area and the high costs for botulinum toxin injection and necessary health staff time.10 The guideline points out that the costs for the treatment of poorly controlled spasticity are unknown and might be high as well, but will mostly be paid by other budgets. In the event of botulinum toxin therapy, in Germany, these barriers could be high costs straining the drug budget of the physician or lack of access to specialists performing botulinum toxin injections. The challenge of separate budgets for inpatient, ambulatory, and long-term nursing care, as mentioned in the UK guideline, has been addressed as a possible barrier for guideline implementation in Germany as well.
We could not identify any study analyzing the treatment of patients with post-stroke spasticity in everyday practice. The Thai study based on a register9 reported some data concerning inpatient treatment for 83 of 136 patients with spasticity. Nearly three-quarters of those patients received therapeutic exercise; 21.8% (elbow flexor) and 23.8% (knee flexor) were treated with oral medications (not further specified), and eight patients received botulinum toxin. The low percentage of patients with intramuscular neurolysis was ascribed to the low number of patients with spasticity of modified Ashworth Scale grade 3 or 4.9
Study limitations and strengths
The study has some limitations. The data cover one insurance fund and one region of Germany, so any generalization of the results has to be done with caution. Because the AOK insures about a third of the German population, we feel confident in making statements for a relevant part of the population. The study is based on claims data that have been collected for administrative purposes. The documented diagnoses were made by the treating physician and could not be confirmed by external clinical examination of the patients. We recruited the stroke patients by hospital diagnosis because acute stroke events are mostly treated in hospital. If stroke was the reason for the hospital stay it is reliably documented in the data because hospitals must document detailed diagnoses for remuneration purposes. Low grades of spasticity may not have been documented if they had required no treatment. Because we had no data on severity of illness, we could not confirm the positive dose-response relationship between physiotherapy resource provision and health outcome in stroke patients as reported by a recently published study.38
However, compared with clinical studies, the scope of the data is a particular strength. Especially in an elderly population with poor health status, as in the case of stroke patients, many patients are probably not able or refuse to participate in clinical trials. Our data allowed us to monitor all persons receiving health care while avoiding selection bias and loss to follow-up. The investigated medical and nonmedical treatment is completely covered by the data. Furthermore, the data base is free of recall bias because it reflects health care utilization independent of memory.
Conclusion
Our study confirms that a relevant percentage of stroke patients suffer from post-stroke spasticity in Germany. To our knowledge, the present study is the first to provide insight into the treatment of post-stroke spasticity in routine health care in comparison with German clinical guideline recommendations. Our data indicate that the provision of physiotherapy in ambulatory care should be increased. According to guideline recommendations, botulinum toxin by injection seems to be underutilized. Further studies, eg, in the setting of ambulant or hospital care, should identify patients with inadequate treatment of post-stroke spasticity and reveal barriers to guideline implementation. Within the framework of continual medical education, general practitioners and neurologists should be informed and motivated to assess their patients with post-stroke spasticity for the need of physiotherapy or medical treatment. Future analyses of routine health care data enable monitoring of the success of efforts to improve guideline implementation.
Acknowledgments
The authors would like to thank the Hesse AOK and Association of Statutory Health Insurance Physicians in Hesse for the opportunity to analyze their data.
Footnotes
Disclosure
The PMV Research Group received an unrestricted grant from Merz Pharmaceuticals GmbH to assess post-stroke spasticity and treatment. The authors of this manuscript did not receive any personal payments from the grantor. The grantor was allowed to comment on a first draft of the manuscript but had no editorial control. The PMV Research Group has received project support from ministries, health insurance funds, associations of statutory health insurance physicians, state institutions, private foundations, and Abbott, Bayer Schering, Lilly, Sanofi-Aventis, and Novo-Nordisk.
References
- 1.World Health Organization The Global Burden of Disease. 2004 Update 2008Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdfAccessed December 3, 2012
- 2.Schinwelski M, Sławek J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol Neurochir Pol. 2010;44:404–411. doi: 10.1016/s0028-3843(14)60300-5. Polish. [DOI] [PubMed] [Google Scholar]
- 3.Caro JJ, Migliaccio-Walle K, Ishak KJ, Proskorovsky I, O’Brien JA. The time course of subsequent hospitalizations and associated costs in survivor of an ischemic stroke in Canada. BMC Health Serv Res. 2006;6:99. doi: 10.1186/1472-6963-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundström E, Terént A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15:533–539. doi: 10.1111/j.1468-1331.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 5.Sommerfeld D, Eek E, Svensson A, Widén Holmqvist L, von Arbin M. Spasticity after stroke. Its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134–139. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 6.Welmer A, von Arbin M, Widén Holmqvist L, Sommerfeld D. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis. 2006;21:247–253. doi: 10.1159/000091222. [DOI] [PubMed] [Google Scholar]
- 7.Wissel J, Schelosky L, Scott J, Christe W, Faiss J, Mueller J. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010;257:1067–1072. doi: 10.1007/s00415-010-5463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins C, Leathley M, Gregson J, Moore A, Smith T, Sharma A. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16:515–522. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 9.Dajpratham P, Kuptniratsaikul V, Kovindha A, et al. Prevalence and management of poststroke spasticity in Thai stroke patients: a multicenter study. J Med Assoc Thai. 2009;92:1345–1360. [PubMed] [Google Scholar]
- 10.Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke. 3rd ed. London, UK: Royal College of Physicians; 2008. [Google Scholar]
- 11.Royal College of Physicians. British Society of Rehabilitation Medicine. Chartered Society of Physiotherapy. Association of Chartered Physiotherapists Interested in Neurology . Spasticity in Adults: Management using Botulinum Toxin. National Guidelines. London, UK: Royal College of Physicians; 2009. [Google Scholar]
- 12.Scottish Intercollegiate Guidelines Network (SIGN) Management of patients with stroke: Rehabilitation, prevention and management of complications, and discharge planning. A national clinical guidelineSIGN publication no 118; 2010Available from: http://www.sign.ac.uk/guidelines/fulltext/118/index.htmlAccessed December 3, 2012
- 13.The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Version 16.03.2008 2008Available from: http://www.eso-stroke.org/recommendations.php?cid=9&sid=1Accessed December 3, 2012
- 14.National Stroke Foundation . Clinical Guideline for Stroke Management 2010. Melbourne, Australia: National Stroke Foundation; 2010. [Google Scholar]
- 15.The Management of Stroke Rehabilitation Working Group VA/DoD Clinical practice guideline for the management of stroke rehabilitation. Version 2.0 2010Available from: http://www.healthquality.va.gov/stroke/stroke_full_221.pdfAccessed December 3, 2012
- 16.Diener HC, editor. Guidelines for Diagnostics and Therapy in Neurology. Stuttgart, Germany: Georg Thieme; 2008. German. [Google Scholar]
- 17.Berger K, Kolominski-Rabas P, Heuschmann P, Keil U. Frequency of stroke in Germany. Deutsch Med Wochenschr. 2000;125:21–25. doi: 10.1055/s-2007-1023879. German. [DOI] [PubMed] [Google Scholar]
- 18.Günster C. Care of stroke in Germany – incidence, hospital readmission, and risk of care reflected by routine data. In: Günster C, Klose J, Schmacke N, editors. [Health Services Research 2011] Stuttgart, Germany: Schattauer; 2011. German. [Google Scholar]
- 19.van den Bussche H, Berger K, Kemper C, Barzel A, Glaeske G, Koller D. Incidence, relapse, nursing care dependency and mortality of stroke in Germany, a secondary analysis of statutory insurance claims data. Akt Neurol. 2010;37:131–135. German. [Google Scholar]
- 20.Lundström E, Smits A, Borg J, Terént A. Four-fold increase in direct cost of stroke survivors with spasticity compared with stroke survivors without spasticity. The first year after the event. Stroke. 2010;41:319–324. doi: 10.1161/STROKEAHA.109.558619. Available from: http://stroke.ahajournals.org/content/41/2/319.short. [DOI] [PubMed] [Google Scholar]
- 21.German Institute of Medical Documentation and Information . [Systematic Index. International Statistical Classification of Diseases and Related Health Problems 10th Revision, German Modification] Köln, Germany: Deutscher Ärzte-Verlag; 2008. [Google Scholar]
- 22.Ihle P, Köster I, Herholz H, Rambow-Bertram P, Schardt T, Schubert I. Sample survey of persons insured in statutory health insurance institutions in Hessen – concept and realisation of person-related data base. Gesundheitswesen. 2005;67:638–645. doi: 10.1055/s-2005-858598. German. [DOI] [PubMed] [Google Scholar]
- 23.Swart E, Ihle P, Klug S, Lampert T. Gute Praxis Sekundärdatenanalyse (GPS)–Revision nach grundlegender Überarbeitung [Good Practice Secondary Data Analysis (GPS) – Revised Version] Gesundheitswesen. 2008;70:54–60. doi: 10.1055/s-2007-1022529. German. [DOI] [PubMed] [Google Scholar]
- 24.Tolonen H, Salomaa V, Torppa J, Sivenius J, Immonen-Räihä P, Lehtonen A. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cariovasc Prev Rehabil. 2007;14:380–385. doi: 10.1097/01.hjr.0000239466.26132.f2. [DOI] [PubMed] [Google Scholar]
- 25.Kirkman MA, Mahattanaakul W, Gregson BA, Mendelow AD. The accuracy of hospital discharge coding for hemorrhagic stroke. Acta Neurol Belg. 2009;109:114–119. [PubMed] [Google Scholar]
- 26.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17:20–26. doi: 10.1002/pds.1518. [DOI] [PubMed] [Google Scholar]
- 27.Efron B, Tibshirani RJ. An introduction to the Bootstrap. Boca Raton, London, New York, Washington DC: Chapman and Hall/CRC; 1993. [Google Scholar]
- 28.National Association of Statutory Health Insurance Physicians . [Uniform Value Scale (EBM)] Ulm, Germany: Deutscher Ärzte-Verlag; 2008. German. [Google Scholar]
- 29.Hoffmann F, Icks A. Diabetes prevalence based on health insurance claims: large differences between companies. Diabet Med. 2011;28:919–923. doi: 10.1111/j.1464-5491.2011.03305.x. [DOI] [PubMed] [Google Scholar]
- 30.Welmer A, Widén Holmqvist L, Sommerfeld D. Location and severity of spasticity in the first 1–2 weeks and at 3 and 18 months after stroke. Eur J Neurol. 2010;17:720–725. doi: 10.1111/j.1468-1331.2009.02915.x. [DOI] [PubMed] [Google Scholar]
- 31.Wissel J, auf dem Brinke M, Hecht M, et al. Botulinum toxin in the treatment of adult spasticity. An interdisciplinary German 10-point consensus 2010. Nervenarzt. 2011;82:481–495. doi: 10.1007/s00115-010-3172-8. German. [DOI] [PubMed] [Google Scholar]
- 32.Wissel J, Olver J, Sunnerhagen KS. Navigating the poststroke continuum of care. J Stroke Cerebrovasc Dis. Jul 4, 2011. [Epub ahead of print.] [DOI] [PubMed]
- 33.Centers for Disease Control and Prevention Outpatient rehabilitation among stroke survivors – 21 States and the District of Columbia, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:504–507. [PubMed] [Google Scholar]
- 34.Francisco GE, Yablon SA, Schiess MC, Wiggs L, Cavalier S, Grissom S. Consensus panel guidelines for the use of intrathecal baclofen therapy in poststroke spastic hypertonia. Top Stroke Rehabil. 2006;13:74–85. doi: 10.1310/tsr1304-74. [DOI] [PubMed] [Google Scholar]
- 35.Sheean G, Lannin N, Turner-Stokes L, Rawicki B, Snow B. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: international consensus statement. Eur J Neurol. 2010;17(Suppl 2):74–93. doi: 10.1111/j.1468-1331.2010.03129.x. [DOI] [PubMed] [Google Scholar]
- 36.Günther J. Muscle relaxants. In: Schwabe U, Paffrath D, editors. [Drug Prescription Report 2009] Heidelberg, Germany: Springer; 2009. [Google Scholar]
- 37.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 38.Haines TP, Kuys S, Clarke J, Morrison G, Bew P. Dose-response relationship between physiotherapy resource provision with function and balance improvements in patients following stroke: a multi-centre observational study. J Eval Clin Pract. 2011;17:136–142. doi: 10.1111/j.1365-2753.2010.01380.x. [DOI] [PubMed] [Google Scholar]


