Abstract
Coincident with climate shifts and anthropogenic perturbations, the highly voracious jumbo squid Dosidicus gigas reached unprecedented northern latitudes along the NE Pacific margin post 1997–98. The physical or biological drivers of this expansion, as well as its ecological consequences remain unknown. Here, novel analysis from both bulk tissues and individual amino acids (Phenylalanine; Phe and Glutamic acid; Glu) in both gladii and muscle of D. gigas captured in the Northern California Current System (NCCS) documents for the first time multiple geographic origins and migration. Phe δ15N values, a proxy for habitat baseline δ15N values, confirm at least three different geographic origins that were initially detected by highly variable bulk δ15N values in gladii for squid at small sizes (<30 cm gladii length). In contrast, bulk δ15N values from gladii of large squid (>60 cm) converged, indicating feeding in a common ecosystem. The strong latitudinal gradient in Phe δ15N values from composite muscle samples further confirmed residency at a point in time for large squid in the NCCS. These results contrast with previous ideas, and indicate that small squid are highly migratory, move into the NCCS from two or more distinct geographic origins, and use this ecosystem mainly for feeding. These results represent the first direct information on the origins, immigration and habitat use of this key “invasive” predator in the NCCS, with wide implications for understanding both the mechanisms of periodic D. gigas population range expansions, and effects on ecosystem trophic structure.
Introduction
Range expansion of species has been linked to recent climate change [1] and has potentially important negative consequences for the population dynamics of native species and trophic structure and biodiversity of entire ecosystems [2]. In the NE Pacific, the most dramatic range expansion, coincident with the 1997–1998 El Niño of the Century, was documented for an r-selected invertebrate species, the jumbo squid (Dosidicus gigas) [3]. As early as 1934, D. gigas were occasionally observed in waters as far as 40°N [4], [5], but their occurrence far surpassed this latitude in 1997, when they were recorded in Oregon (45°N) [3], and subsequently in the Gulf of Alaska in 2005, although they remained most abundant in waters offshore of the USA and British Columbia from 2002 to 2009 [3], [6], [7]. Multiple drivers have been proposed for this dramatic expansion, including climate change, the expansion and shoaling of the oxygen minimum zone (OMZ) in the California Current [6], [7], and the depletion of tuna and bill fish populations in the eastern tropical Pacific [8]. While this species' tolerance to a wide range of temperatures and oxygen concentrations likely facilitates shifts in distribution and abundance [9], the specific environmental triggers, underlying drivers, and geographic origins of the expanding populations remain unclear.
Range expansion and high abundance of D. gigas has likely also altered trophic structure of the Northern California Current System (NCCS), as this species is an opportunistic voracious predator with high growth rates and energetic demands [10]. It is also the largest ommastrephid (maximum mantle length >1.5 m) with the highest potential of fecundity (>30 million eggs) of any cephalopod species [11]. In the NCCS, it consumes a wide range of prey items (n>100), including hake (Merluccius productus), sardine (Sardinops sagax) and rockfish (Sebastes spp.) [12], [13], all species of high economic importance. Temporal variation in recruitment and movement of this opportunistic and aggressive predator can induce considerable variation in population dynamics and life history of prey species [10]. In British Columbia, for example, regional declines in hake abundance have been attributed to D. gigas predation [14]. However, a major puzzle regarding D. gigas range expansions is that studies to date have not been able to detect long-distance migration using bulk stable isotope ratios or electronic tagging, implying that medium to large squid move only within modest latitudinal distances (∼4° of latitude) [9], [15]. The fundamental mechanism for D. gigas expansions is therefore unclear: it is unknown whether D. gigas expanded its range into the NCCS through migration, or through passive dispersal of egg masses and paralarvae.
To investigate origins, movement and habitat use of D. gigas inhabiting the NCCS, we analyzed stable isotope ratios of nitrogen (δ15N) from bulk tissue samples (gladii and muscle) and conducted compound specific isotope analysis of individual amino acids (CSIA-AA) from squid captured there (Figure 1). Natural stable isotope abundances can provide time-integrated information about the trophic position of species in a given habitat [16], [17]. In D. gigas, bulk isotope ratios from consecutive sections along gladii (internal shell) can also track ontogenetic shifts [15]. Isotope ratios from muscle indicate recent assimilated diet (<2 months), while consecutive sections along gladii represent a continuous dietary record throughout the period of time in which the gladius was formed [18], [19]. Because baseline δ15N values (i.e. values from primary producers) are heterogeneous across marine ecosystems, ocean basins and latitudes [20], movement can be detected along gladii as squid grow if D. gigas feed while moving between isotopically distinct habitats. However, bulk isotope analysis often cannot distinguish between shifting habitat baselines vs. trophic position.
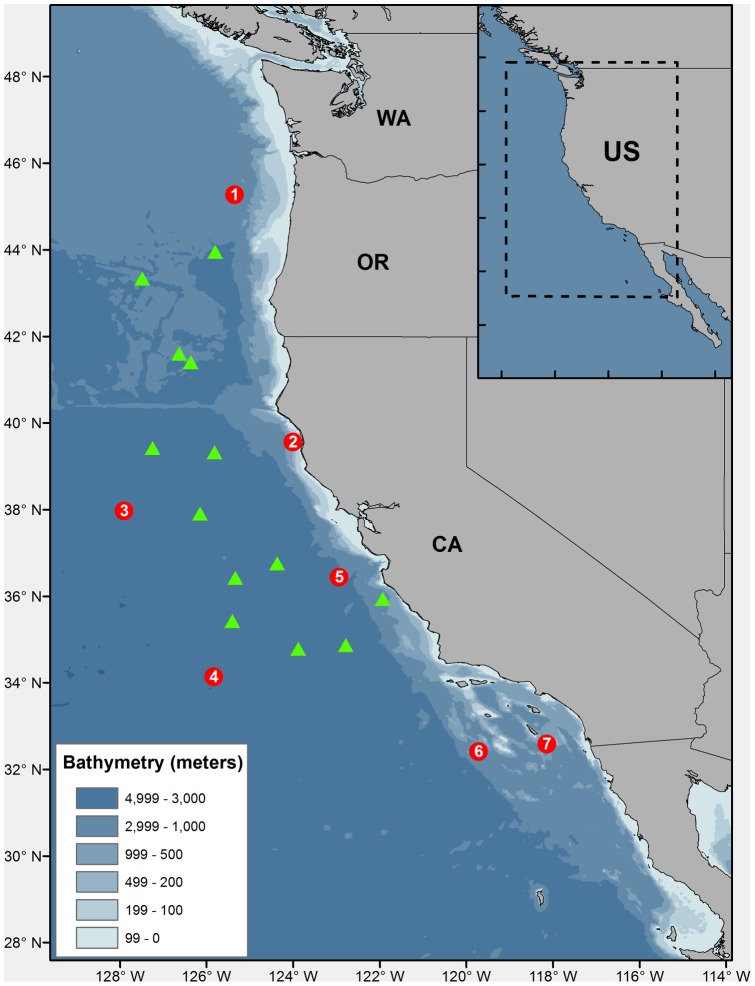
Figure 1. Sampling sites of Dosidicus gigas in 2008.
Circles indicate the seven stations where squid were captured and pre-selected for stable isotope analysis. Triangles indicate other locations where squid were successfully captured.
CSIA-AA decouples these two factors by specifically identifying approximate baseline δ15N values for an organism, as well as shifts in trophic position [21]–[23]. Analyzing tissues from prey and taxa near the base of the food web is therefore not necessary, because trophic position can be estimated using specific source (e.g. Phenylalanine)- and trophic (Glutamic acid)- amino acid δ15N values coupled with equations tested under control experiments [22]. In this way, CSIA-AA has been used to document variation in specific amino acids of yellowfin tuna in the eastern tropical Pacific, indicating a relatively constant trophic position across different latitudes and longitudes, along with clear spatial differences in baseline δ15N values for these highly mobile predators [24], [25]. Nevertheless, CSIA-AA has so far been applied to a few taxa only, and never for cephalopods. Our study applies CSI-AA for the first time in squid, coupling its unique information with traditional bulk δ15N analysis to decrypt the mechanism for maintaining D. gigas populations in the NCCS during 2008.
Results and Discussion
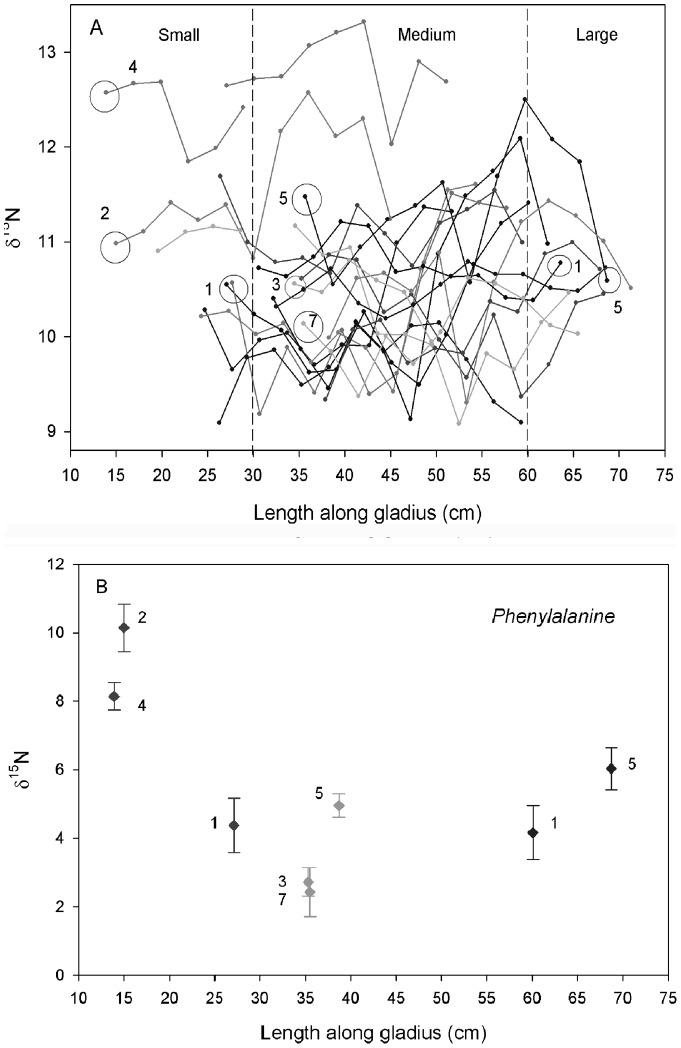
A total of 115 squid (mean mantle length (1SD) = 61.3 cm (8.1)) were collected from 20 stations in the NCCS, Sept – Nov, 2008 (Figure 1). Seven stations were selected a priori representing the maximum latitudinal and longitudinal range, for subsequent analysis of squid tissues. For these seven stations, mantle lengths ranged from 31–75 cm (mean (1SD) = 62.2 cm (9.6)), and a total of 210 gladii sections from 19 individual squid were analyzed (δ15N ranged from 9‰–13.3‰). Mean bulk gladii δ15N values differed for each squid between the seven stations (Generalized Linear Model between stations F6,12 = 31.01, p<0.001, and squid nested by station F12, 190 = 14.39, p<0.001; model R2 = 0.64). Comparisons of bulk δ15N values along the length of gladii for these 19 squid revealed generally greater variation at small sizes (<30 cm gladii length (GL), Variance = 0.95) than larger sizes (Variance 0.8 for medium sizes: 30–60 cm GL, and 0.3 for large sizes: >60 cm GL; Figure 2A). Associated with this variation between squid at a given size was ontogenetic variation within individual squid as they grew, though the specific pattern differed between squid. Some squid had decreasing or increasing δ15N values as they grew, and some showed no discernible trend (Fig. 2A). These results partially differed from a previous study of D. gigas in the NE Pacific in which δ15N were also highly variable along gladii, but generally increased as a function of size [15]. These authors interpreted this variation as evidence of opportunistic predation with squid feeding at higher trophic levels as they grew in the same ecosystem.
Figure 2. Relationship between gladii length and δ15N values (‰).
(A) Relationship between length along gladii and bulk δ15N values from all proostracum sections (n = 210) of 19 squid collected at seven stations (numbers correspond to station numbers in Figure 1). Each line represents an individual squid, and dots along the line indicate δ15N values at each 3 cm section mean length. Open circles indicate the gladii sections of specific squid selected for further CSIA-AA. (B) Phe δ15N values at a given gladii length for the selected gladii sections at small, medium and large sizes.
In contrast, the δ15N patterns along gladii in the present study indicate migration into the NCCS from multiple origins, rather than ontogenetic shifts in diet of resident squid within the NCCS. In particular for small squid, arm size and the chitinous beak pose constraints on prey size so that small squid feed on small prey and at low trophic levels [26]. Given the same diet composition and habitat, δ15N from multiple small individuals should be homogenous, as opposed to the observed heterogeneous values (Figure 2A). Our further CSIA-AA analysis allows a first direct examination to distinguish long-distance migration between regions of different δ15N baseline values, from changes in trophic position as an explanation for change in bulk δ15N values. Specifically, Phenylalanine (Phe) is the most stable of the so called “source” amino acids, and provides a direct estimate for δ15N value at the base of food webs, while glutamic acid (Glu) is widely used as the most reliable “trophic” amino acid, central to cycling of nitrogen, and so, strongly 15N-enriched relative to diet [21]–[23]. Here, measured Phe δ15N values from a subset of gladii sections at lengths <30 cm confirm different baseline values, and therefore distinct geographic origins of small squid (Figure 2B).
Given these different Phe δ15N values for squid at small sizes and 35 cm GLs (Figure 2B), our CSI-AA data can suggest specific geographic regions for the origins of northern D. gigas population. Phe δ15N values represent a proxy for baseline δ15N values, as previously demonstrated in multiple environments [21], [23], [27]. However, while Phe δ15N values derived from primary production remain relatively unchanged with trophic transfer [21], [22], only approximate links to either bulk algal or local nitrate δ15N values can be made because (a) the exact offset between Phe and bulk algal δ15N values is not yet precisely known [22], and (b) if inorganic nitrogen is not completely utilized at a given location or time period where squid fed, additional isotopic fractionation between local nitrate and algal δ15N values would be expected [28].
Considering the known geographic patterns in baseline δ15N value variation in the N Pacific [29]–[31], one geographic origin likely includes waters of northern Baja California, Mexico, since Phe δ15N values (4–8‰) correspond to δ15N-NO3 − values from 100–200m depths between 28°N and 35°N (5–8.5‰) [29]. Direct observations support this, as small- to medium-sized D. gigas (21–35.9 cm MLs) have been collected offshore of N Baja California, Mexico (30 July, 2006 at 29°N and 116°W; R. I. Ruiz-Cooley, squid collection). A second possible source region, corresponding to the highest Phe δ15N values observed in our squid (10‰), includes regions of active denitrification such as waters of the southern Baja California Peninsula (e.g., 22–23°N; δ15N-NO3 − = 9-13‰) [29]. Lastly, a third possible source region corresponds to the lowest Phe δ15N values (2–3‰) measured (see squid at 35 cm GLs; Figure 2B). These low values are not consistent with δ15N-NO3 − values in the CCS, British Columbia or eastern tropical Pacific [30], While it cannot be ruled out that these squid values may reflect regions of replete surface nitrate (where strong isotope fractionation favors the lighter isotope [28]), such depleted δ15N values likely indicate a source region where N fixation influences primary production δ15N values. Both ocean temperatures and the influence of N fixation increase rapidly moving offshore from the main CCS into the edge of the more oligotrophic gyre [32]. Therefore these values could be consistent with a source region in much warmer offshore waters to the west, perhaps near the boundary of the north Pacific Gyre (NPSG) system.
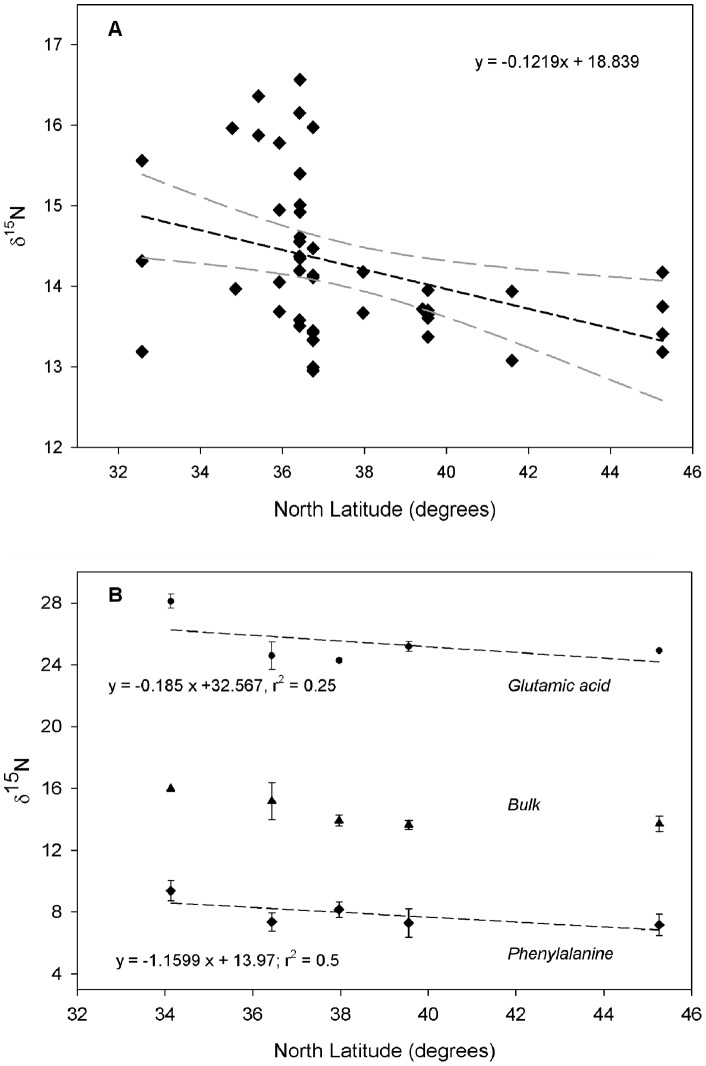
In contrast to the high variation in δ15N for small squid, bulk δ15N and Phe δ15N values for squid larger than 60 cm exhibited low variation (Figure 2A, B), indicating a common feeding area for large animals. Further, Phe and Glu δ15N values in composite muscle tissue samples exhibited a latitudinal gradient, with progressively decreasing values as latitude increased from 33°N to 46°N (Figure 3). Following the approach of Popp et al [24], the observation that change in Glu δ15N parallels that in Phe δ15N indicates that the decreasing bulk δ15N observed with increasing latitude is due to spatial change in baseline isotopic values, and not to shifts in trophic position. Furthermore, Glu-Phe Δ15N values, representing a direct proxy for relative trophic position, remained constant as a function of latitude (Glu-Phe Δ15N ranged from 16.1 to 18.7‰; Figure 3B). The decreasing trend in both bulk and CSIA-AA is strongly consistent with the expected latitudinal gradient in baseline δ15N values in the NCCS, as documented by multiple sample types, including upper water column of NO3 −, organic matter from both sediment traps and sediments collected from southern California to Oregon [30], [31], and also from D. gigas muscle collected in 2006 [33]. This baseline δ15N attenuation with latitude is fundamentally driven by California Undercurrent northward transport of elevated δ15NO3 − waters originating in the strongly denitrifying eastern tropical Pacific [30], [31], [34], while lower salinity and higher dissolved O2 surface waters of the California Current are transported equatorward [35]. The fact that a δ15N latitudinal gradient for three muscle tissue isotopic measurements (bulk, Phe and Glu) all match the expected gradient in baseline δ15N values for the NCCS, indicates residence of large squid at specific latitudes for time periods long enough to integrate local marine biochemical processes.
Figure 3. Dosidicus gigas latitudinal variation in δ15N values (‰).
(A) Latitudinal variation in bulk δ15N values (n = 45) from muscle and (B) amino acid compound specific data from composite muscle samples. Sample size for each composite sample in a given stations is: n = 3 squid for stations 1, 2 and 5; n = 2 squid for station 3; n = 1 squid for station 4. Mean and one standard deviation are shown for Phenylalanine (the main source amino acid;⧫), Glutamic acid (the main trophic amino acid; ▪), and bulk (▴) δ15N. Dashed lines indicate linear regressions: (A) δ15Nbulk, r2 = 0.14, p<0.01; (B) δ15NGlutamic acid, p = 0.4 and δ15NPhenylalanine, p = 0.1.
Such “regional” residence for this highly mobile predator might seem unexpected based on prior tagging experiments. Squid larger than 70 cm ML have been shown to move within a particular ecosystem (i.e., only within the central Gulf of California) over distances as great as180 km in one week [9], and mainly between inshore-offshore waters in the NCCS [36]. However, maturity data and size frequencies of 870 jumbo squid support the idea that jumbo squid move seasonally and large mature females leave the NCCS abruptly in the fall to spawn in Mexican waters [13]. If large squid do move seasonally, why would Phe δ15N in muscle track the expected latitudinal δ15N baseline gradient? A likely explanation unifying all these observations is that the δ15N isotopic equilibration rates for squid muscle may be faster than previous estimates of 2 months [19]. This rate was calculated based on a simple dilution model and available growth rates, from the population in the Gulf of California. Since only the large morph is found in the NCCS [11], our data suggest that new equilibration rates are needed once growth rates are available for this system. Furthermore, once females leave the NCC, they possibly remain in spawning areas until they release all their eggs and die. D. gigas are intermittent, multiple spawners and can release 5–30 million eggs they produce over a lifetime (oviducts can hold approximately 1.2 million eggs at any one time) within an approximate period of 3 to 4 months) [37]. The energy cost for spawning is expected to be high, and no long-distance migrations would be expected during its spawning period.
This study demonstrates how δ15N from bulk tissues coupled with CSIA-AA of a highly dynamic r-selected species can reveal distinct geographic origins and ontogenetic changes in habitat use and residence patterns. Our data indicate that D. gigas inhabiting waters from 32° to 45°N latitude (Figure 1) in fact emigrated from multiple regions prior to reaching their largest size. Once in the NCCS, these large squid remained and fed in specific regions, at least long enough to integrate baseline isotopic values. Fisheries data indicate that other species of ommastrephids (e.g., Illex sp.) may be capable of migrating over long distances for spawning, reproduction and feeding [38]. Our study provides the first direct evidence that small ommastrephids do migrate, likely over long-distances and between ecosystems that are biochemically distinctive. For D. gigas, there are no direct observations of mass migrations, but multiple strandings recorded along the coasts of California, Oregon, and Canada [39] are consistent with the idea that such migrations do occur.
Together, these results have important implications for understanding the influence of climate change on range expansions. The appearance and high abundance of D. gigas in CA occurred post-1997 and 2002, years of warm SST in the eastern tropical Pacific [8]. To date, paralarvae of jumbo squid have not been found in waters north of the U.S.-Mexico border [40], and laboratory experiments have confirmed that eggs can successfully develop only at water temperatures between 15°C and 25°C [41]. Because larvae and small sized-squid have a lower thermal tolerance than large squid [42], we hypothesize that wider distribution for spawning eggs and higher success in egg development during anomalously high temperature events (e.g., El Niño events), coupled with migration of smaller squid, promote range expansions of D. gigas. Under this scenario, small-sized squid migrate toward the NCCS to feed actively, as shown by stomach content analysis [13], grow until they mature, and likely return to warmer waters to spawn multiple times in regions that include coastal and offshore Mexican waters along the Baja California peninsula, and possibly the edge of NPSG. Finally, because D. gigas is a short lived species (∼1.5 years), the fact that our data show multiple origins for individuals collected from the NCCS in a single year (2008), coupled with observations that the species remained abundant in this ecosystem for multiple years (2002–2009) [8], [13], indicates that multiple immigrations over time were required to maintain the NCCS population. This hypothesis conflicts with a previous one which proposed a sustained northern population with a lack of multiple invasions [8]. Both expansion of OMZ areas and increased fisheries pressure on top predators have been proposed as factors that triggered D. gigas range expansions. However, both these factors have continued to accelerate, while the population of D. gigas in the NE Pacific has contracted since 2009 [43] These observations, together with the data documented here, strongly support the idea that El Niño years in 1997 and 2002 were most responsible for triggering the multiple migrations that lead to the recent and long-lasting range expansion of D. gigas.
Materials and Methods
Squid were collected by jigging under a bright light during hour-long nightly oceanographic stations, each of which began one hour after sunset. Squid sampling was part of a larger cetacean and ecosystem assessment survey of the NCCS conducted by NOAA [44]. Within an hour of capture, squid were measured and frozen at −20°C. Additional processing was conducted post survey. For each, the gladius was extracted, measured and the proostracum (that portion of the gladius formed by the ostracum [45]) was cut transversely every 3 cm sections and prepared for stable isotope analysis (SIA) following Ruiz-Cooley et al. [15]. Details of bulk δ15N analysis (‰) are provided elsewhere. A GLM with nested factors (each squid nested by station) was used to compare the effect of sampling location for a given squid δ15N values on gladii. In addition, squid were classified into three size classes: small (<30 cm GL), medium 30–60 cm GLs) and large (>60 cm GLs), to investigate ontogenetic variation. Muscle tissue from the mantle (of 19 squid from the 7 geographic extreme stations plus an additional 28 squid randomly selected from all remaining stations) was prepared for SIA following Ruiz-Cooley and Gerrodette [33]. Linear regression was performed to examine the relationship between latitude and δ15N values.
For CSIA-AA, 3.5–4 mg of a subset of gladii sections (n = 8) at small, medium and large squid lengths (see Figure 2) and five muscle composite samples (each composed of different squid randomly selected for a given station; see caption Figure 3B) were analyzed following previously published protocols [46]. Briefly, individual AA isotopic analyses were made on acid hydrolysates (6 N HCl, 100 ml, 20 h) of homogenized material, following the formation of isopropyl-TFA derivatives. Derivatives were analyzed on a Thermo Trace Ultra GC, fitted with a Agilent DB-5 column (50 m×0.32 mm i.d. ×0.52 um film thickness), in line with an oxidation furnace and reduction furnace, and linked to a Finnigan DeltaPlus XP mass spectrometer. All samples were derivatized using an accompanying AA external standard for which authentic δ15N values of each AA were determined offline, to monitor the accuracy of the instrument; an internal standard (Nor-Leu) was also added to each sample hydrolysate. Samples were injected in quadruplicate, bracketed by standards, and sample δ15N values were corrected based on a running average of known standard values through a run. Reproducibility for individual AA values was typically better than 1‰.
Acknowledgments
We thank J. Barlow, A. Henry, J. Cotton, K. Baltz, the Protected Resources Divison (SWFSC, NMFS, NOAA), and the officers and crew of the MCARTHURII for their support in the collection of specimens. We thank V. Atudorei (University of New Mexico) and E. Gier (UCSC) for support for stable isotope analysis. We thank Corey Sheredy (PRD-SWFSC) for her support on map construction. We also thank our anonymous reviewers for constructive comments.
Funding Statement
The authors have no funding or support to report.
References
- 1. Parmesan C (2006) Ecological and Evolutionary Responses to Recent Climate Change. Annu Rev Ecol Evol S 37: 637–669. [Google Scholar]
- 2. Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401: 464–467. [Google Scholar]
- 3. Pearcy WG (2002) Marine nekton off Oregon and the 1997–98 El Nino. Progr Oceanogr. 54: 399–403. [Google Scholar]
- 4. Croker RS (1937) Further notes on the jumbo squid, Dosidicus gigas . Calif Fish Game 23: 246–247. [Google Scholar]
- 5. Clark FN, Phillips JB (1936) Commercial use of the jumbo squid, Dosidicus gigas . Calif Fish Game 22: 143–144. [Google Scholar]
- 6. Rodhouse PG (2008) Large-scale range expansion and variability in ommastrephid squid populations: A review of environmental links. Calif Coop Ocean Fish Invest Rep 49: 83–89. [Google Scholar]
- 7. Bograd SJ, Castro CG, Di Lorenzo E, Palacios DM, Bailey H, et al. (2008) Oxygen declines and the shoaling of the hypoxic boundary in the California Current. Geophys Res Lett 35: L12607. [Google Scholar]
- 8. Zeidberg LD, Robison BH (2007) Invasive range expansion by the jumbo squid, Dosidicus gigas, in the eastern North Pacific. Proc Natl Acad Sci USA 104: 12948–12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilly WF, Markaida U, Baxter CH, Block BA, Boustany A, et al. (2006) Vertical and horizontal migrations by the jumbo squid Dosidicus gigas revealed by electronic tagging. Mar Eco Progr Ser 324: 1–17. [Google Scholar]
- 10. Rodhouse PG, Nigmatullin CM (1996) Role as consumers. Philos Tran R Soc Lond B 351: 1003–1022. [Google Scholar]
- 11. Nigmatullin CM, Nesis KN, Arkhipkin AI (2001) A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish Res 54: 9–19. [Google Scholar]
- 12. Field JC, Baltz K, Phillips AJ, Walker WA (2007) Range expansion and trophic interactions of the jumbo squid, Dosidicus gigas, in the California Current. Calif Coop Ocean Fish Invest Rep 48: 131–146. [Google Scholar]
- 13.Field JC, Elliger C, Baltz K, Gillespie GE, Gilly WF, et al. (2012) Foraging ecology and movement patterns of jumbo squid (Dosidicus gigas) in the California Current System. Deep Sea Res. 10.1016/j.dsr2.2012.09.006.
- 14. Holmes J, Cooke K, Cronkite G (2008) Interactions between jumbo squid (Dosidicus gigas) and Pacific hake (Merluccius productus) in the northern California Current in 2007. Calif Coop Ocean Fish Invest Rep 49: 86–89. [Google Scholar]
- 15. Ruiz-Cooley RI, Villa EC, Gould WR (2010) Ontogenetic variation of δ13C and δ15N recorded in the gladius of the jumbo squid Dosidicus gigas: geographic differences. Mar Ecol Prog Ser 399: 187–198. [Google Scholar]
- 16. Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48: 1135–1140. [Google Scholar]
- 17. Hobson KA, Welch HE (1992) Determination of Trophic Relationships within a High Arctic Marine Food Web Using δ13C and δ15N Analysis. Mar Ecol Prog Ser 84: 9–18. [Google Scholar]
- 18. Staaf DJ, Ruiz-Cooley RI, Elliger C, Lebaric Z, Campos B, et al. (2010) Ommastrephid squids Sthenoteuthis oualaniensis and Dosidicus gigas in the eastern Pacific show convergent biogeographic breaks but contrasting population structures. Mar Ecol-Prog Ser 418: 165–U587. [Google Scholar]
- 19. Ruiz-Cooley RI, Markaida U, Gendron D, Aguinga S (2006) Stable isotopes in jumbo squid (Dosidicus gigas) beaks to estimate its trophic position: comparison between stomach contents and stable isotopes. J Mar Biol Assoc UK 86: 437–445. [Google Scholar]
- 20.Somes CJ, Schmittner A, Galbraith ED, Lehmann MF, Altabet MA, et al.. (2010) Simulating the global distribution of nitrogen isotopes in the ocean. Global Biogeochem Cy 24.
- 21. McClelland JW, Montoya JP (2002) Trophic Relationships and the Nitrogen Isotopic Composition of Amino Acids in Plankton. Ecology 83: 2173–2180. [Google Scholar]
- 22. Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, et al. (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr 7: 740–750. [Google Scholar]
- 23. Hannides CCS, Popp BN, Landry MR, Graham BS (2009) Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnol Oceanogr 54: 50–61. [Google Scholar]
- 24.Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott MJ, et al. (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of protenaceous amino acids. In: Dawson TD, Siegwolf RTW, editors. Stable isotopes as indicators of ecological change. 173–190.25.
- 25. Olson RJ, Popp BN, Graham BS, Lopez-Ibarra GA, Galvan-Magana F, et al. (2010) Food-web inferences of stable isotope spatial patterns in copepods and yellowfin tuna in the pelagic eastern Pacific Ocean. Prog Oceanogr 86(1–2): 124–138. [Google Scholar]
- 26. Shchetinnikov AS (1989) Food spectrum of Dosidicus gigas (Oegopsida) in the ontogenesis. Zool Zh 68: 28–39. [Google Scholar]
- 27.Sherwood OA, Lehmann MF, Schubert CJ, Scott DB, McCarthy MD (2011) Nutrient regime shift in the western North Atlantic indicated by compound-specific δ15N of deep-sea gorgonian corals. Proc Natl Acad Sci USA. 10.1073/pnas.1004904108. [DOI] [PMC free article] [PubMed]
- 28.Montoya JP (2008) Natural Abundance of 15N in Marine Planktonic Ecosystems. In: Michener R, Lajtha K, editors. Stable Isotopes in Ecology and Environmental Science. 176–201.
- 29.Sigman DM, Granger J, DiFiore PJ, Lehmann MM, Ho R, et al.. (2005) Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin. Global Biogeochem Cy 19.
- 30. Altabet MA, Pilskaln C, Thunell R, Pride C, Sigman D, et al. (1999) The nitrogen isotope biogeochemistry of sinking particles from the margin of the Eastern North Pacific. Deep Sea Res 46: 655–679. [Google Scholar]
- 31. Kienast SS, Calvert SE, Pedersen TF (2002) Nitrogen isotope and productivity variations along the northeast Pacific margin over the last 120 kyr: Surface and subsurface paleoceanography. Paleoceanography 17: 1055. [Google Scholar]
- 32. Karl DM (1999) A Sea of Change: Biogeochemical Variability in the North Pacific Subtropical Gyre. Ecosystems 2: 181–214. [Google Scholar]
- 33. Ruiz-Cooley RI, Gerrodette T (2012) Tracking large-scale latitudinal patterns of δ13C and δ15N along the eastern Pacific using epi-mesopelagic squid as indicators. Ecosphere 3: 1–17. [Google Scholar]
- 34. Liu K-K, Kaplan IR (1989) The eastern tropical Pacific as a source of 15N-enriched Nitrate in seawater off southern California. Limnol Oceanogr 34: 820–830. [Google Scholar]
- 35. Reid JL, Roden JG, Wyllie JG (1958) Studies of the California Current system. Calif Coop Oceanic Fish Invest Report 6: 27–56. [Google Scholar]
- 36.Stewart JS, Gilly WF, Field JC, Payne JC (2012) Onshore – offshore movement of jumbo squid (Dosidicus gigas) on the continental shelf. Deep Sea Res.
- 37. Nigmatullin CM, Markaida U (2008) Oocyte development, fecundity and spawning strategy of large sized jumbo squid Dosidicus gigas (Oegopsida: Ommastrephinae). J Mar Biol Assoc UK 1: 1–13. [Google Scholar]
- 38.Boyle PR, Rodhouse PG (2005) Cephalopods: ecology and fisheries. Blackwell Science Ltd. 452 p.
- 39. Braid H, Deeds J, DeGrasse S, Wilson J, Osborne J, et al. (2012) Preying on commercial fisheries and accumulating paralytic shellfish toxins: a dietary analysis of invasive Dosidicus gigas (Cephalopoda Ommastrephidae) stranded in Pacific Canada. Mar Biol 159: 25–31. [Google Scholar]
- 40.Watson W, Manion SI (2011) Ichthyoplankton, paralarval cephalopod, and station data for surface (Manta) and oblique (bongo) plankton tows for California Cooperative Oceanic Fisheries Investigations Survey and California Current Ecosystem Survey cruises in 2008. ed NMFS-SWFSC-481 NTM.
- 41. Staaf DJ, Zeidberg LD, Gilly WF (2011) Effects of temperature on embryonic development of the Humboldt squid Dosidicus gigas . Mar Ecol Prog Ser 441: 165–175. [Google Scholar]
- 42. Pimentel M, Trübenbach K, Faleiro F, Boavida-Portugal J, Repolho T, et al. (2012) Impact of ocean warming on the early ontogeny of cephalopods: a metabolic approach. Mar Biol 159: 2051–2059. [Google Scholar]
- 43. Bjorkstedt R, Goericke S, McClatchie S, Weber E, Watson W, et al. (2011) State of the California Current 2010–2011. Regionally variable responses to a strong (but fleeting?) La Niña. Calif Coop Ocean Fish Invest Rep 52: 36–68. [Google Scholar]
- 44. Barlow J, Forney KA (2007) Abundance and population density of cetaceans in the California Current ecosystem. Fish Bull 105: 509–526. [Google Scholar]
- 45.Bizikov VA (1991) A new method of squid age determination using the gladius. In Jereb, P, Ragonese S, Boletzki SV, editors. Squid age determimnations using statoliths. NTR-ITTP. 39–51.
- 46. McCarthy MD, Benner R, Lee C, Fogel M (2007) Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim Cosmochim Ac 71: 4727–4744. [Google Scholar]