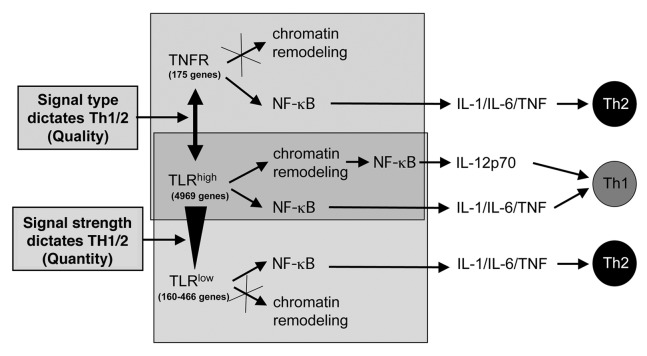
Figure 1. Qualitative and quantitative differences in dendritic cell maturation affect TH1/TH2 polarization. Dendritic cell (DC) maturation can be initiated by various types of pattern recognition receptors, such as Toll-like receptors (TLRs), or by the receptors for various pro-inflammatory cytokines, such as the tumor necrosis factor α (TNFα) receptor TNFR. The genetic signatures resulting from TNFR-conveyed and weak TLR-conveyed signals are remarkably small and highly similar to each other, sharing a common pro-inflammatory component. When DC maturation is triggered by TNFR or weak TLR signals (TLRlow), the transcription factor NFκB can rapidly bind to the promoter region of genes coding for interleukin (IL)-1, IL-6 and TNFα. This type of DC maturation promotes TH2-cell polarization. In response to these signals, no chromatin remodeling at the IL-12-coding gene promoter occurs to allow for the binding of NF-κB. In contrast, strong and prolonged TLR (TLRhigh) signals are required to allow for chromatin remodeling at promoter region of the IL-12-coding gene and hence for the (delayed) binding of NFκB, resulting in the maturation of TH1-polarizing DCs. This model integrates findings indicating that both DC maturation signal type (quality) and intensity (quantity) influence can TH1 vs. TH2-cell polarization.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.