Fig. 3.
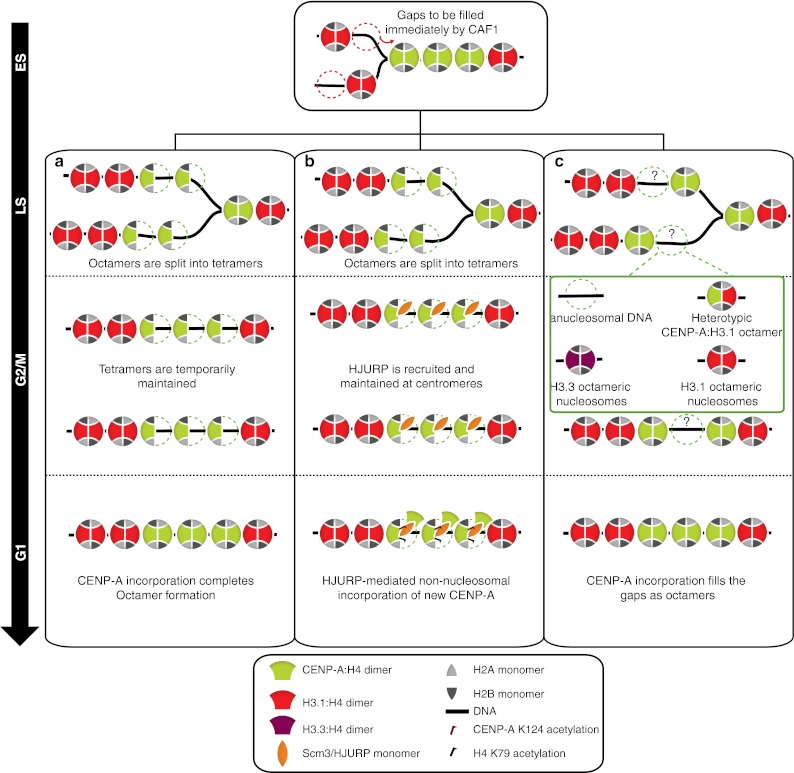
Proposed structural dynamics of centromeric nucleosomes throughout the cell cycle. a An octamer to tetramer transition model in which passage of the replication fork splits the pre-existing octameric CENP-A nucleosomes into tetramers allowing equal inheritance of the epigenetic mark to daughter strands. In this model, HJURP exclusively is found at the centromere in G1 and mediates the reconstruction of octamers by incorporation of new CENP-A. b The tetramer to octamer transition model. CENP-A:H4: HJURP complex is recruited to the centromeric chromatin in G1 and is assumed to associate to the pre-existing CENP-A tetramers extra-nucleosomally. Posttranslational modifications (PTMs) of tetrameric CENP-A nucleosomes along with the presence of HJURP impede the stable incorporation of new CENP-A:H4 dimers into nucleosomes. In late G1/early S, action certain proteins chaperones and remodeling complexes is proposed to facilitate the tetramer to octamer transition by affecting the PMTs of histones and chromatin structure resulting in tetramer to octamer transition. Likewise to the previous model, passage of the replication fork splits the octamers to the tetramers. The tetramers are further stabilized by the reassociation of HJURP in G2 and presumed to be maintained in mitosis to form the kinetochore plate. c. In an alternative model, where CENP-A exists in octameric nucleosomes throughout the cell cycle, gaps generated as a result of the passage of the replication fork will be either maintained or could be transiently filled with multiple possible placeholder structures. In this model, HJURP is also found at the centromere exclusively in G1 to participate in new CENP-A assembly
