Fig. 1.
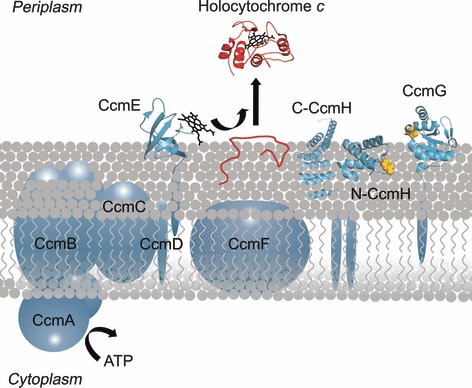
The cytochrome c maturation System I. The Ccm proteins (in blue) are all integral membrane proteins or are membrane-anchored with soluble domains in the periplasm (with the exception of CcmA, which hydrolyses ATP in the cytoplasm). The structures of the soluble domains of CcmE, CcmG and the N-terminal domain of CcmH have been solved and are shown on the periplasmic side (the Protein Data Bank accession numbers are 1LIZ [53], 2B1K [54] and 2HL7 [21], respectively). The structure of a paralog of the C-terminal domain of CcmH, NrfG, is also shown (Protein Data Bank accession number: 2E2E [23]). The holocytochrome c shown is from Paracoccus denitrificans (Protein Data Bank accession number: 155C). CcmH in Escherichia coli is a fusion of two proteins that occur separately in other organisms (CcmH and CcmI, labeled N-CcmH and C-CcmH). The apocytochrome c protein is shown in red, as is the holocytochrome c produced when heme (shown in black) becomes covalently attached. The cysteine residues, assumed to be involved in reducing the –CXXCH– motif in the apocytochrome, are shown in yellow.
