Figure 2.
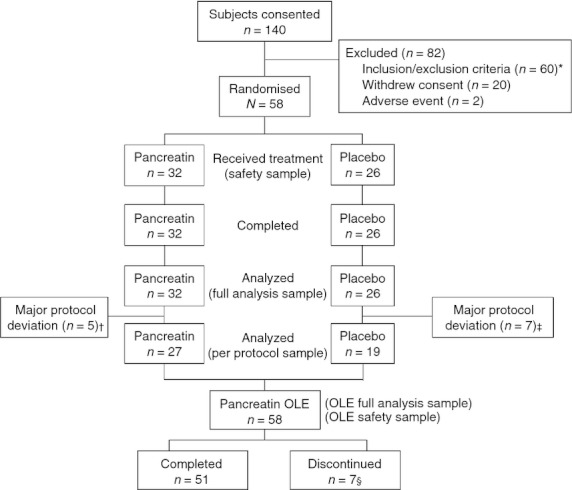
Patient disposition. OLE, open-label extension. *Majority did not meet the interim inclusion criterion of CFA <80%. †Insufficient exposure to study medication during double-blind phase (n = 3); insufficient treatment compliance (n = 1); deviation from inclusion criterion of CFA <80% (n = 3). ‡Insufficient exposure to study medication during double-blind phase (n = 3); insufficient treatment compliance (n = 2); insufficient essential efficacy data (n = 1); use of prohibited/prior concomitant medication (n = 1). §Withdrew consent (n = 3), adverse events (n = 2), lost to follow-up (n = 1), and administrative reasons (n = 1).
