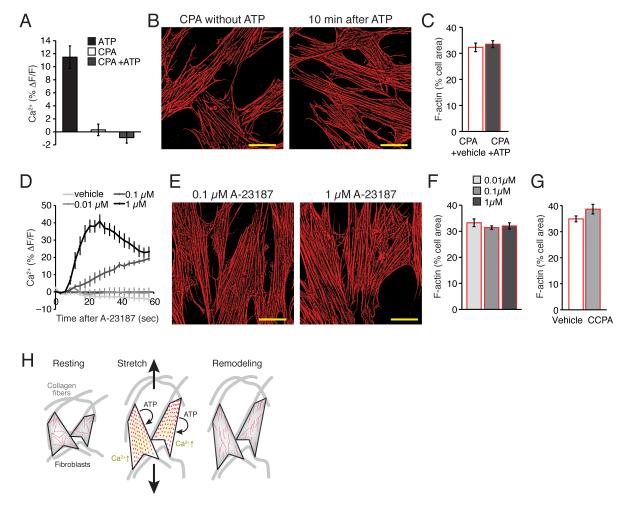
Fig. 2. Increase in cytosolic Ca 2+ is required, but not sufficient for purine-induced cytoskeletal reorganization.
(A) Ca2+ increase by ATP (100 μM) is completely blocked by a pretreatment of CPA (20 μM). N = 6, p = 0.35, t test between CPA and CPA+ATP. (B) The presence of CPA (20 μM) blocks F-actin (red) disassembly by ATP. Scale bars, 30 μm. (C) A summary histogram of actin disassembly at 10 min after vehicle or ATP (100 μM) application in the presence of CPA (20 μM). N = 6, p > 0.1, t tests. (D) Ca2+ increases by A-23187 (0, 0.01, 0.1, & 1 μM). N = 6, p < 0.001, ANOVA. (E) A Ca2+ ionophore A-23187 failed to induce F-actin (red) disassembly. Scale bars, 30 μm. (F) A summary histogram of F-actin disassembly at 10 min after application of A-23187 (0, 0.01, 0.1, & 1 μM). N = 11, p > 0.5, ANOVA. (G) A summary histogram of F-actin disassembly at 10 min after application of CCPA (10 μM). N = 7-8, p > 0.05 t tests compared to vehicle. (H) Proposed model. Fibroblast cells in situ are attached to collagen fibers of extracellular matrix. Increase of mechanical tension by tissue stretch causes ATP release, which in autocrine fashion activates own purinergic receptors. Purinergic activation triggers Ca2+ signaling and disassembly of polymerized actin. This transient disassembly enables the cells to undergo longer lasting morphological remodeling.