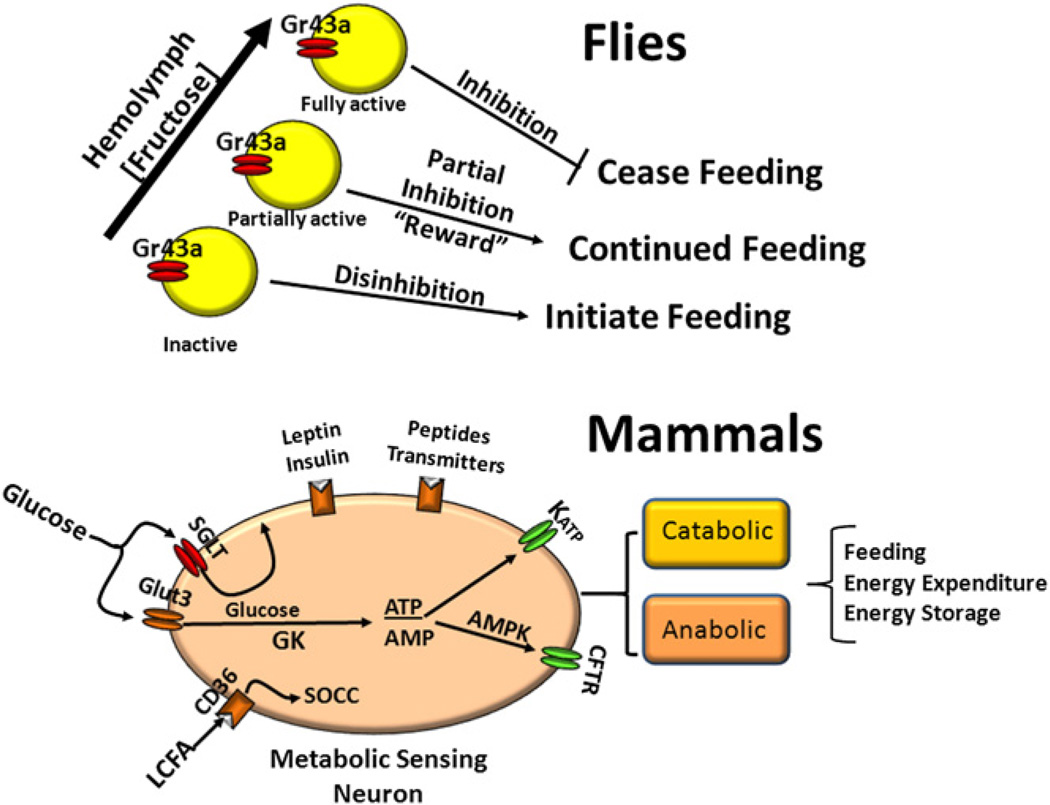
Figure 1. Hypothetical Comparison of Feeding and Energy Homeostasis Systems in Flies and Mammals.
In flies, hemolymph concentrations of fructose are detected in the brain by fructose-sensing neurons expressing Gr43a (yellow). The neurons are inactive at low and activated at high fructose concentrations. This negative feedback system promotes feeding when neurons are inactive and halts feeding above a critical fructose concentration. At intermediate concentrations, activation of Gr43a neurons also provides a reward function that promotes continued feeding. In mammals, there is a complex set of nutrient (glucose, long-chain fatty acid [LCFA]), hormonal (leptin, insulin) and neural inputs from the periphery, which are integrated at the level of membrane potential and alter neuronal activity in different sets of catabolic and anabolic neurons. Glucose is sensed by transport through the Glut3 transporter, phosphorylation by glucokinase (GK) followed by production of ATP, which acts on the ATP-sensitive K+ (KATP) channel to activate glucose excited neurons. When glucose levels fall, AMP is produced, activating neuronal AMP-activated kinase (AMPK) and the cystic fibrosis transmembrane receptor (CFTR), which then stimulates glucose-inhibited neurons. The sodium-glucose cotransporter (SGLT) generates an electrogenic potential that activates glucose excited neurons independently of glucose metabolism. LCFA interact with the fatty-acid translocator/CD36 receptor (CD36) to activate store-operated calcium channels (SOCC), leading to inhibition or excitation of subsets of metabolic sensing neurons (MSN).