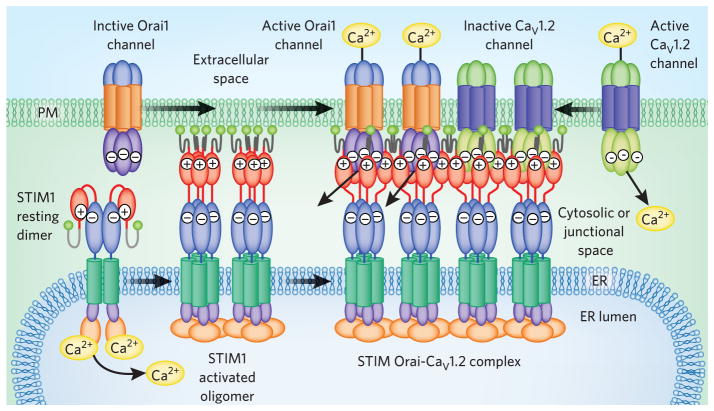
Figure 3.
Multiple targets for STIM1. STIM1 interacts with both Orai1 and CaV1.2 channels, forming a large junctional complex within which STIM1 activates Orai1 channels and inhibits CaV1.2 channels. At rest, STIM1 is likely a dimer with its CAD domain (red) masked by internal binding to CC1 (blue; see Fig. 1). Luminal Ca2+ depletion causes STIM1 oligomerization and umasking of CAD acidic residues that bind basic residues on Orai1, trapping and activating its channel. The interaction with CaV1.2 channels requires the same STIM1-CAD domain but has yet to be characterized. Both channel targets appear to coexist within the same ER-PM junctional complex.