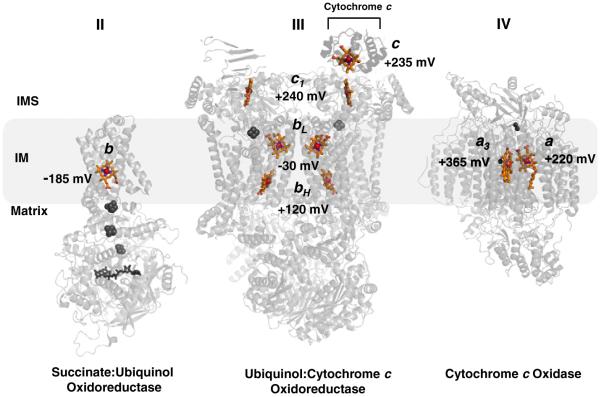
Fig. 1.
Distribution of hemes b, c, and a, with respective midpoint potentials, in respiratory complexes II, III, and IV in the mitochondria. Note the general trend of increasing redox potential from the b heme of Complex II to the a3 heme of Complex IV, the site of the terminal electron acceptor and O2 reduction. Other cofactors in the complexes are grayed for clarity: Complex II; FAD, [2Fe–2S], [4Fe–4S], [3Fe–4S]: Complex III; Rieske 2Fe–2S: Complex IV; binuclear CuA, CuB. Complex III is shown as a dimer with cytochrome c docked to one of the subunit. References used for midpoint potentials: Complex II [21]; Complex III [124]; Complex IV [125]. The figure was generated using PDB accession numbers: 1YQ3, 3CX5 and 2OCC.