Fig. 4.
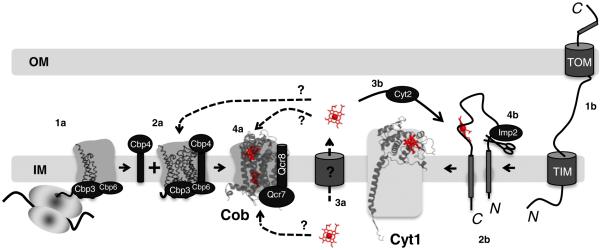
Hemylation of cytochrome b and cytochrome c1 polypeptides in eukaryotic bc1 complex. Translation of COB mRNA initiates bc1 complex biogenesis (1a). Cob forms a ternary complex with the Cbp3/Cbp4/Cbp6 proteins independent of the mitochondrial ribosome (2a). Following transport of newly synthesized heme across the IM (3a), this is a possible point at which heme is inserted into the apo-cytochrome b from the IMS side of the inner membrane. This insertion could occur prior to, in conjunction with, or post Qcr7 and Qcr8 additions (4a). Transport across the IM would not be required if the product heme from ferrochelatase is deposited into the IM (see Fig. 6a). Hemylation of Cob could also occur directly from the matrix side. Apo-cytochrome c1 is synthesized in the cytosol and must be imported into the mitochondria through the inner and outer membrane translocases (1b). A bipartite signaling sequence directs insertion into the IM (2b). Following transport of newly synthesized heme across the IM, the heme lyase, Cyt2, participates in the covalent attachment of heme c1 to apocytochrome c1 (3b). The final step in holocytochrome c1 maturation is cleavage by Imp2 to remove the N-terminal membrane anchor, resulting in an Nout–Cin topology (4b). PDB accession number: 3CX5 was used to generate the figure.
