Abstract
Of the several aquaporin (AQP) water channels expressed in the central nervous system, AQP4 is an attractive target for drug discovery. AQP4 is expressed in astroglia, most strongly at the blood–brain and brain–cerebrospinal fluid barriers. Phenotype analysis of AQP4 knockout mice indicates the involvement of AQP4 in three distinct processes: brain water balance, astroglial cell migration and neural signal transduction. By slowing water uptake into the brain, AQP4 knockout mice manifest reduced brain swelling and improved outcome in models of cytotoxic cerebral oedema such as water intoxication, focal ischaemia and meningitis. However, by slowing the clearance of excess water from brain, AQP4 knockout mice do worse in models of vasogenic oedema such as brain tumour, abscess and hydrocephalus. AQP4 deficient astroglial cells show greatly impaired migration in response to chemotactic stimuli, reducing glial scar formation, by a mechanism that we propose involves AQP4-facilitated water flux in lamellipodia of migrating cells. AQP4 knockout mice also manifest increased seizure threshold and duration, by a mechanism that may involve slowed K+ uptake from the extracellular space (ECS) following neuroexcitation, as well as ECS expansion. Notwithstanding challenges in drug delivery to the central nervous system and their multiplicity of actions, AQP4 inhibitors have potential utility in reducing cytotoxic brain swelling, increasing seizure threshold and reducing glial scar formation; enhancers of AQP4 expression have potential utility in reducing vasogenic brain swelling. AQP4 modulators may thus offer new therapeutic options for stroke, tumour, infection, hydrocephalus, epilepsy and traumatic brain and spinal cord injury.
Keywords: AQP4, water transport, transgenic mouse, brain oedema, cell migration, epilepsy
Introduction
Excess accumulation of brain water occurs in a wide range of brain disorders such as stroke, tumour, infection, hydrocephalus and traumatic injury. Brain oedema results in elevated intracranial pressure, potentially leading to brain ischaemia, herniation and death. Current treatments for brain oedema, which include hyperosmolar agents and surgical decompression, have changed little since their introduction more than 80 years ago. As described in this review, the water transporting protein aquaporin-4 (AQP4) provides an important route for water movement between blood and brain, and between brain and CSF compartments. Distinct from its involvement in transcellular water transport and brain swelling, AQP4 involvement in astroglial cell migration and neural signal transduction has been discovered. Each of these distinct roles of AQP4 suggests specific therapeutic strategies, such as AQP4 inhibition to reduce brain oedema and seizure susceptibility, and enhance neuronal regeneration following brain injury. This review assesses experimental evidence in support of the potential indications of AQP modulators in brain therapy, and discusses challenges in their implementation. Our focus will be on AQP4, because of its potential in translational developments, with brief discussion of two other AQPs, AQP1 and AQP9, that are expressed in brain.
Aquaporins in normal brain
AQP4, the principal brain water channel, is expressed in glia at the borders between major water compartments and brain parenchyma (Nielsen et al., 1997; Rash et al., 1998) including astroglial foot processes (blood–brain barrier), glia limiting membrane (subarachnoid cerebrospinal fluid–brain interface) as well as ependyma and subependymal astroglia (ventricular cerebrospinal fluid–brain interface). This pattern of expression suggests that AQP4 controls the flow of water into and out of the brain, and may thus play a key role in brain oedema. Two AQP4 splice variants are expressed in brain, termed M1 and M23, which can form homo- and heterotetramers, respectively, which in turn assemble into square lattices of tetramers within astroglial cell plasma membranes (Yang et al., 1996; Verbavatz et al., 1997; Furman et al., 2003; Rash et al., 2004; Silberstein et al., 2004). The relative abundance of M1 vs. M23 isoforms determines the size of orthogonal arrays, which can contain up to several hundred tetramers (Furman et al., 2003), and possibly influences AQP4 single channel water permeability (Silberstein et al., 2004). Compared with non-interacting AQP4 tetramers, orthogonally inter-connected tetramers are proposed to allow more efficient anchoring of AQP4 to intracellular proteins such as alpha syntrophin. For example, for polarization of AQP4 expression in astroglial endfeet each non-interacting AQP4 tetramer would require its own intracellular anchor, whereas an orthogonal arrangement might require only one anchor per raft.
The other major brain water channel, AQP1, is found primarily in the ventricular-facing (apical) membrane of choroid plexus epithelium, where it plays a role in the formation of cerebrospinal fluid (Oshio et al., 2005). Interestingly, AQP1 is not expressed in normal brain vascular endothelial cells, although it is ubiquitously present in peripheral vascular endothelia (Nielsen et al., 1993; Dolman et al., 2005). The observations that brain endothelial cells cultured in the absence of astroglia express AQP1, as do brain tumour endothelia (which are not surrounded by glia), suggest that astroglia produce signals to inhibit AQP1 expression in the brain vascular endothelium.
It has been suggested that AQP9 is also expressed in the brain (Badaut and Regli, 2004). AQP9 immunoreactivity was detected in a subpopulation of glia called tanycytes, in some astroglia, and in glucose-sensitive catecholaminergic neurons. Because AQP9 also transports small molecules, including glycerol and lactate, it has been suggested that AQP9 may participate in brain energy metabolism (Badaut and Regli, 2004). AQP9 expression in mitochondria (Amiry-Moghaddam et al., 2005) provides further evidence for the involvement of AQP9 in energy metabolism. However, recent studies have questioned the presence and physiological role of AQP9 in brain. A major problem with AQP9 research is the lack of good anti-AQP9 antibodies for immunohistochemical experiments, as evidenced by the comparable AQP9 immunoreactivity detected in the brains of wildtype and AQP9 null mice (Rojek et al., 2007). Also, recent functional studies and theoretical arguments argue against water channel expression and function in mitochondria (Yang et al., 2006b).
Aquaporin regulation studies
Although the pattern of water channel expression in the brain provides important clues about AQP function, further information has been obtained from regulation studies. In general, AQP4 expression becomes upregulated in brain astroglia after injury including trauma, tumour and haemorrhage (Papadopoulos and Verkman, 2007). In human astrocytomas, AQP4 expression in tumours correlates with the presence of brain swelling on magnetic resonance scans (Saadoun et al., 2002), raising the possibility that AQP4 participates in the formation and/or absorption of brain tumour oedema. Interestingly, dexamethasone, which is commonly used clinically to reduce brain tumour oedema, does not alter AQP4 expression, with the AQP4 promoter lacking steroid response elements. Therefore, AQP4 modulators may act synergistically with corticosteroids in reducing brain tumour oedema. Increased expression of AQP4 in reactive and malignant astroglia suggests that AQP4 may play further roles in the functions of these cells, such as in glial scar formation and the growth/spread of astroglial tumours. AQP1, which is normally found in the choroid plexus, becomes upregulated in choroid plexus tumours, which are associated with increased cerebrospinal fluid production (Longatti et al., 2006). There is also evidence that AQP9 immunoreactivity changes in response to injury (Badaut and Regli, 2004), though as mentioned above there are significant concerns about the specificity of anti-AQP9 antibodies. In general, regulation studies have provided indirect evidence about the functions of AQPs in the brain. Most of our knowledge about AQP functions comes from studies using AQP null mice.
Roles of aquaporins in brain
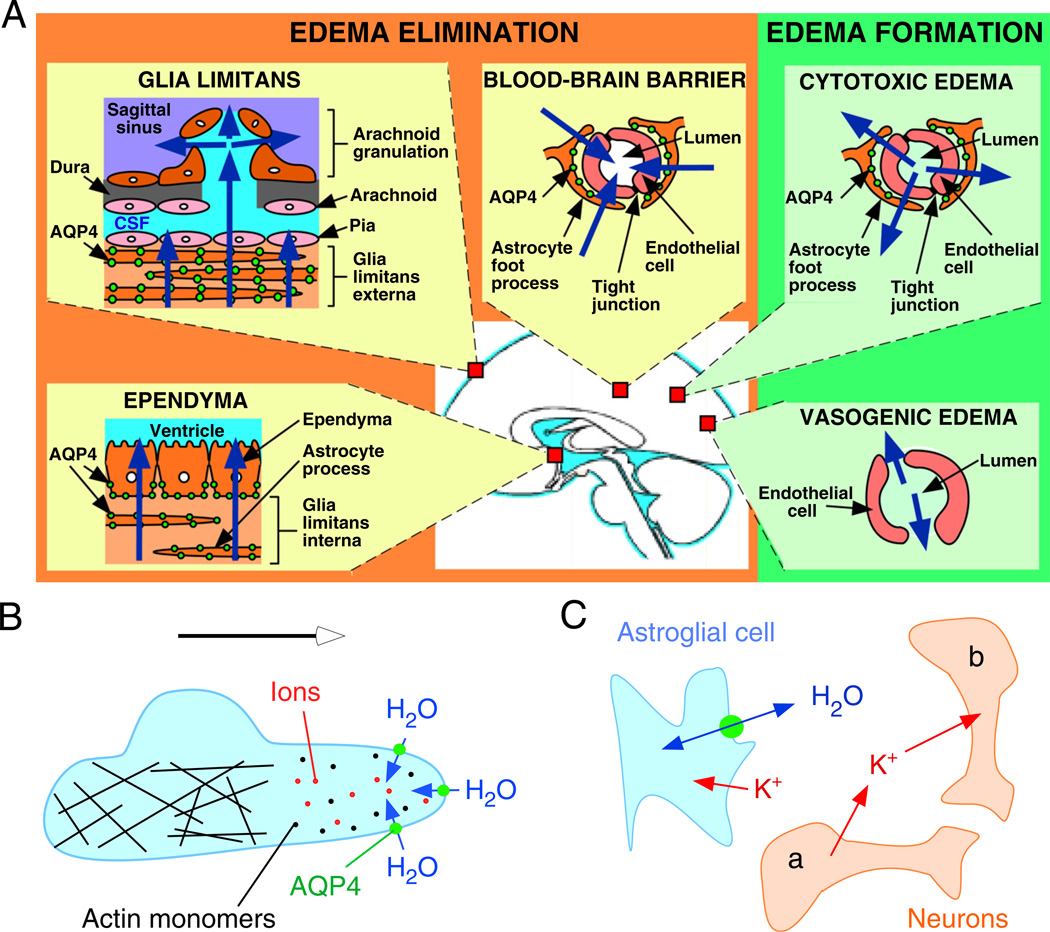
AQPs have three major functions in the brain: control of brain water balance, cell migration and neuronal excitability (summarized in Fig. 1). The movement of water into and out of the brain parenchyma is primarily controlled by AQP4, with AQP1 contributing to the formation of cerebrospinal fluid by the choroid plexus. AQP4 facilitates the migration of reactive astroglia towards a site of injury, and may also accelerate the migration of malignant astroglia. AQP1 facilitates the migration of endothelial cells, thus accelerating angiogenesis in growing tumours. AQP4 controls the size of the extracellular space (ECS) and extracellular K+ kinetics, which are important components of neuronal excitability. These three major AQP functions are discussed in more detail below.
Fig. 1.
Schematic depicting three distinct roles of AQP4 (green circles) in brain function: (A) brain water balance, (B) astroglial cell migration and (C) neuronal excitation. (A) Green. Routes of oedema formation in the two types of brain oedema (cytotoxic — through AQP4, vasogenic — through interendothelial spaces). Orange. Oedema fluid is eliminated by AQP4 through the glial limitans into subarachnoid CSF, through ependyma and sub-ependymal astroglia into ventricular CSF, and through astroglial pericapillary foot processes into blood. (B) AQP4 polarizes to the leading edge of migrating astroglia and accelerates cell migration. AQP4 facilitates water entry into lamellipodial protrusions in response to intracellular hyperosmolality produced by actin depolymerization and ion influx. (C) AQP4 deletion reduces neuroexcitation. Active neurons (neuron a) release K+ into the extracellular space (ECS). Increased extracellular [K+] depolarizes quiescent neurons (neuron b). AQP4 deletion increases ECS volume and reduces astroglial cell K+ reuptake. This buffers the increase in extracellular [K+] by active neuron a, preventing depolarization of quiescent neuron b. See text for further explanations. (See Color Plate 46.1 in color plate section.)
Brain water balance
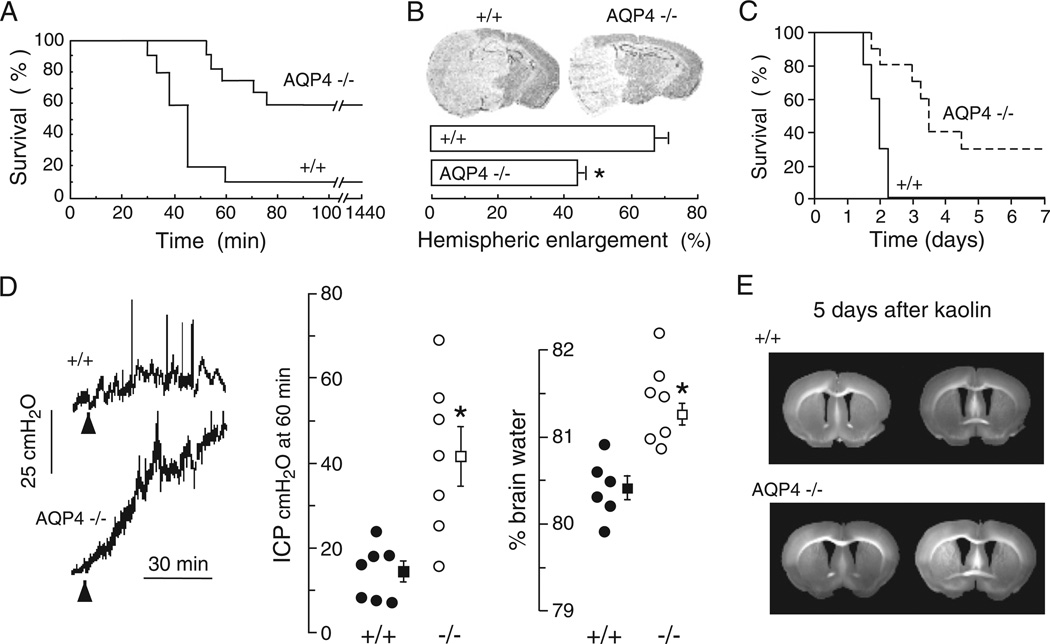
The pattern of AQP4 expression in the brain (at the borders between brain parenchyma and major fluid compartments) as well as regulation studies (correlating AQP4 expression and brain oedema), provide indirect evidence for involvement of AQP4 in brain water balance. Table 1 summarizes evidence supporting involvement of AQP4 in brain water balance. Direct evidence came from experiments showing reduced brain swelling and improved survival in AQP4 null versus wildtype mice after water intoxication (Fig. 2A), reduced hemispheric swelling after focal cerebral ischaemia (Fig. 2B) and improved survival after bacterial meningitis (Fig. 2C) (Manley et al., 2000; Papadopoulos and Verkman, 2005). Reduced brain swelling was also reported in alpha-syntrophin null mice, which secondarily have disrupted brain AQP4 expression (Amiry-Moghaddam et al., 2003a, 2004). According to the Klatzo classification of brain oedema (Klatzo, 1994), these are primarily models of cytotoxic (cell swelling) oedema in which excess water moves from the vasculature into the brain parenchyma through an intact blood–brain barrier (Fig. 1A). The forces driving water flow to form cytotoxic oedema are osmotic, generated in water intoxication by reduced plasma osmolality and in ischaemia by failure of the Na+/K+ ATPase with consequent Na+ and water flow from the intravascular and extracellular compartments into the intracellular compartment. Taken together, these studies suggest that by reducing the flow of water from the vasculature into the brain parenchyma, AQP4 limits the rate of brain water accumulation in cytotoxic oedema. Therefore, AQP4 inhibitors or downregulators are predicted to be powerful anticytotoxic oedema agents.
Table 1.
Summary of studies implicating involvement of AQP4 in brain oedema
| Organism | Evidence | Principal finding | Pathological process | References |
|---|---|---|---|---|
| Human | Indirect | Increased AQP4 expression correlates with oedema | Astrocytoma | Saadoun et al., 2002; Warth et al., 2007 |
| Human | Indirect | Increased AQP4 expression | Brain tumours Subarachnoid haemorrhage | Badaut et al., 2003 |
| Human | Indirect | Increased AQP4 expression | Cerebral infarct | Aoki et al., 2003 |
| Rat | Indirect | Changes in AQP4 expression correlate with oedema | Cerebral ischaemia | Meng et al., 2004; Badaut et al., 2007; Chen et al., 2007 |
| Mouse | Indirect | Changes in AQP4 expression correlate with oedema | Cerebral ischaemia | Ribeiro Mde et al., 2006 |
| Rat | Indirect | Changes in AQP4 expression correlate with oedema | Traumatic brain injury | Kiening et al., 2002; Sun et al., 2003; Chen et al., 2007 |
| Rat | Indirect | Increased AQP4 immunoreactivity | Hyponatremia | Ke et al., 2002 |
| Rat | Indirect | High AQP4 expression correlates with BBB opening | Median forebrain bundle injury | Vizuete et al., 1999 |
| Mouse | Direct | Reduced brain oedema in AQP4 null mice | Water intoxication, cerebral ischaemia | Manley et al., 2000 |
| Mouse | Direct | Reduced brain oedema in alpha syntrophin null mice | Water intoxication, cerebral ischaemia | Amiry-Moghaddam et al., 2003a, 2004 |
| Mouse | Direct | Reduced brain oedema in AQP4 null mice | Bacterial meningitis | Papadopoulos and Verkman, 2005 |
| Mouse | Direct | Increased brain oedema in AQP4 null mice | Brain melanoma, focal cortical freeze, aCSF infusion | Papadopoulos et al., 2004 |
| Mouse | Direct | Increased brain oedema in AQP4 null mice | Brain abscess | Bloch et al., 2005 |
| Mouse | Direct | Increased hydrocephalus in AQP4 null mice | Hydrocephalus | Bloch et al., 2006 |
Fig. 2.
AQP4 deletion reduces brain water accumulation in cytotoxic oedema, but slows removal of excess brain water in vasogenic oedema. (A) Water intoxication model of cytotoxic oedema. Survival of 12 wildtype vs. 12 AQP4 knockout mice after acute water intoxication produced by intraperitoneal water injection (20% body weight). (B) (Top) Ischaemic stroke model of cytotoxic oedema. Brain sections of mice at 24 h after ischaemic stroke produced by permanent middle cerebral artery occlusion. (Bottom) Average hemispheric enlargement expressed as a percentage determined by image analysis of brain sections (7 AQP4+/+ vs. 7 AQP4−/− mice, SEM, * P < 0.0002). (C) Mouse survival (10 AQP4+/+ vs. 10 AQP4−/− mice, P < 0.001) in a bacterial model of meningitis produced by cisternal injection of S. pneumoniae. (D) Reduced elevation in intracranial pressure (ICP, SEM, * P < 0.01) and brain water content (SEM, * P < 0.001) following continuous intraparenchymal infusion of artificial cerebrospinal fluid at 0.5 µL/min. (E) Accelerated progression of hydrocephalus in AQP4 null mice. Coronal sections wildtype and AQP4 null mouse brain at 5 days after kaolin injection. Data from Manley et al., 2000, Papadopoulos et al., 2004 and Bloch et al., 2006.
Subsequent experiments showed that AQP4 also plays a key role in elimination of excess brain water. When the blood–brain barrier becomes disrupted (brain tumour, brain abscess, focal freeze injury), water moves from the vasculature into the ECS of the brain in an AQP4-independent manner down a hydrostatic gradient to form, what was termed by Klatzo (1994), vasogenic oedema. Excess water is eliminated primarily through the glia limiting membrane into the cerebrospinal fluid (Klatzo, 1994). There is increased brain water gain and intracranial pressure in AQP4 null versus wildtype mice with brain tumour, brain abscess, focal cortical freeze injury and after infusion of normal saline directly into brain ECS (Fig. 2D) (Papadopoulos et al., 2004; Bloch et al., 2005), suggesting that vasogenic oedema fluid is eliminated by an AQP4-dependent route (Fig. 1A). Therefore, AQP4 activators or upregulators are predicted reduce brain swelling in pathologies associated with vasogenic brain oedema. Kaolin injection into the cisternal magna obstructs cerebrospinal fluid outflow from the 4th ventricle (Bloch et al., 2006). AQP4 null mice develop more marked hydrocephalus than wildtype mice after kaolin injection, probably due to reduced transependymal water clearance in the AQP4 null mice (Fig. 2E). AQP4 inhibitors may thus provide primary or adjunctive medical therapy in obstructive hydrocephalus.
AQP1 controls water movement from blood vessels into the ventricles in the formation of cerebrospinal fluid. Compared with wildtype mice, in AQP1 null mice the rate of cerebrospinal fluid formation is reduced by about 25%, which was associated with reduced osmotic permeability of the choroid plexus epithelium and a twofold decrease in intracranial pressure (Oshio et al., 2005). Though these changes are modest, and in part related to reduced central venous pressure in AQP1 null mice, it was suggested that AQP1 inhibitors might be useful in the treatment of hydrocephalus or benign intracranial hypertension (pseudotumour cerebri).
Astroglial cell migration
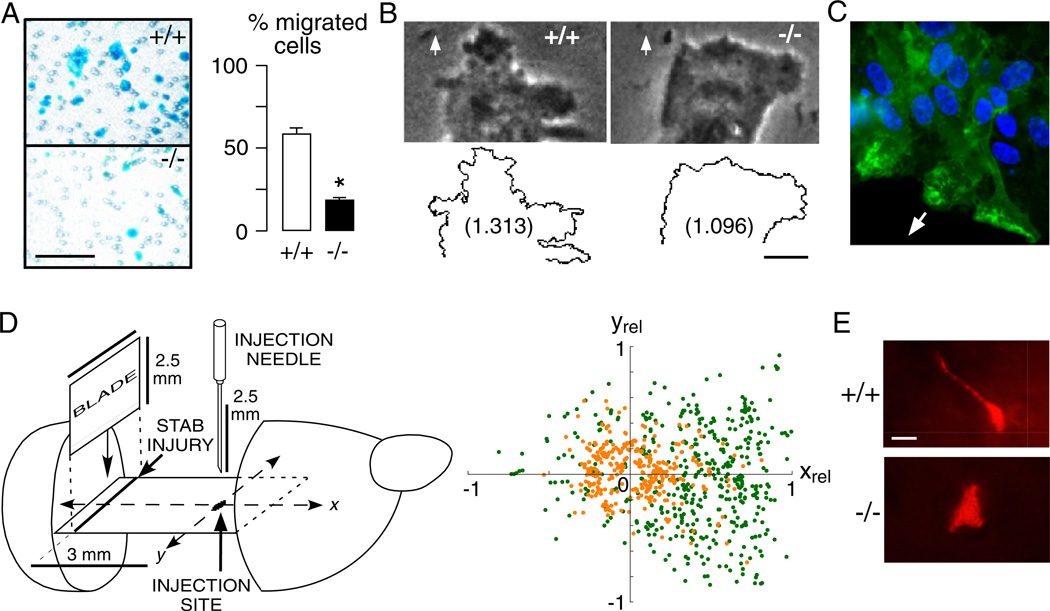
After brain injury, astroglia throughout the brain become reactive and migrate towards the lesion site to form a glial scar (Kastner, 1987; Fawcett and Asher, 1999). Recent evidence suggests that AQP4, which becomes over-expressed in reactive astroglia, facilitates astroglial cell migration (Saadoun et al., 2005b; Auguste et al., 2007). Reduced migration speed was seen in cultured AQP4 null versus wildtype astroglia in transwell (Fig. 3A) and in vitro scratch assays, and was associated with reduced glial scarring in AQP4 null mice after a cortical stab injury. Further studies showed that increased cell migration in AQP expressing cells is a general phenomenon independent of AQP type and cell type (Saadoun et al., 2005a; Hara-Chikuma and Verkman, 2006; Hu and Verkman, 2006; Levin and Verkman, 2006; Loitto et al., 2007). AQP1 facilitates migration in endothelial cells, Chinese Hamster Ovary, Fisher Rat Thyroid, renal proximal tubule cells, as well as malignant melanoma and breast carcinoma cells in vitro and in vivo. AQP3 facilitates migration in skin keratinocytes and corneal epithelial cells. Two possible mechanisms involved in AQP-dependent cell migration have been proposed (Papadopoulos et al., 2008). According to the first hypothesis, AQPs may facilitate the movement of water across the cell membrane at the leading edge of the cell in response to rapid changes in intracellular osmolality that occur in this region. This mechanism is consistent with the increased number of cell membrane protrusions in lamellipodia of AQP expressing vs. non-expressing cells (Fig. 3B), as well as the polarization of AQPs to the front end of migrating cells (Fig. 3C) (Saadoun et al., 2005a, b). According to the second hypothesis, AQPs facilitate rapid changes in cell shape that are required for migrating cells to squeeze through the irregular ECS and to push apart stationary cells in their way. This mechanism, which would be undetectable in standard 2-dimensional migration assays, may explain why differences in migration between wildtype and AQP4 null astroglia are more marked in brain in vivo than in culture. Figure 3D summarizes an in vivo migration study of astroglia towards a stab wound in brain, which showed faster and more directional migration in wildtype vs. AQP4 null astroglia. Consistent with a major role for AQP4 in rapid cell shape changes during migration, more elongated wildtype vs. AQP4 null astroglia were seen as they migrated in intact brain (Fig. 3E). Whatever the mechanism, the ability of AQPs to accelerate cell migration has important therapeutic implications. AQP4 inhibitors may reduce glial scarring, which is the major impediment to neuronal regeneration after injury in the central nervous system (Fawcett and Asher, 1999). Inhibition of AQP4 (and AQP1) may reduce tumour infiltration, for example the infiltration of malignant glioma cells into the surrounding brain. By reducing endothelial cell migration, AQP1 inhibitors are predicted to inhibit angiogenesis, thus reducing tumour growth.
Fig. 3.
AQP4 facilitates astroglial migration in vitro and in brain. (A) Left. Boyden chamber migration assay showing AQP4+/+ and AQP4−/− astroglia (blue) after scraping off the non-migrated cells. Astroglia were plated on the top chamber of porous transwell filter (2.8 × 104/cm2) and were allowed to migrate for 6 h towards 10% FBS as chemoattractant. Bar, 100 µm. Right. Summary of migration experiments (SEM, * P < 0.001). (B) Phase contrast micrographs (Top) and outline (Bottom) of the leading end of a migrating AQP4+/+ and AQP4−/− astroglial cell in the in vitro wound assay. Arrows show direction of migration. Numbers are fractal dimensions (larger number denotes more irregular cell membrane). Bar 10 µm. (C) AQP4 protein (green) polarization to the front end of migrating astroglia in a wound assay. Arrow shows direction of migration. (D) Left. Stab injury/cell injection model of astroglial migration in mouse brain. Two days before cell injection, a stab was created as shown. Cultured AQP4+/+ and AQP4+/+ astroglia were fluorescently labelled and injected as indicated. Right. Locations of migrating fluorescently stained AQP4+/+ (green) and AQP4−/− (orange) astroglia. The x-axis is relative distance between injection and stab injury sites (xrel). (E) High magnification fluorescence micrographs of migrating AQP4+/+ and AQP4−/− astroglia. Bar 5 µm. Data from Saadoun et al., 2005b and Auguste et al., 2007. (See Color Plate 46.3 in color plate section.)
Neural signal transduction
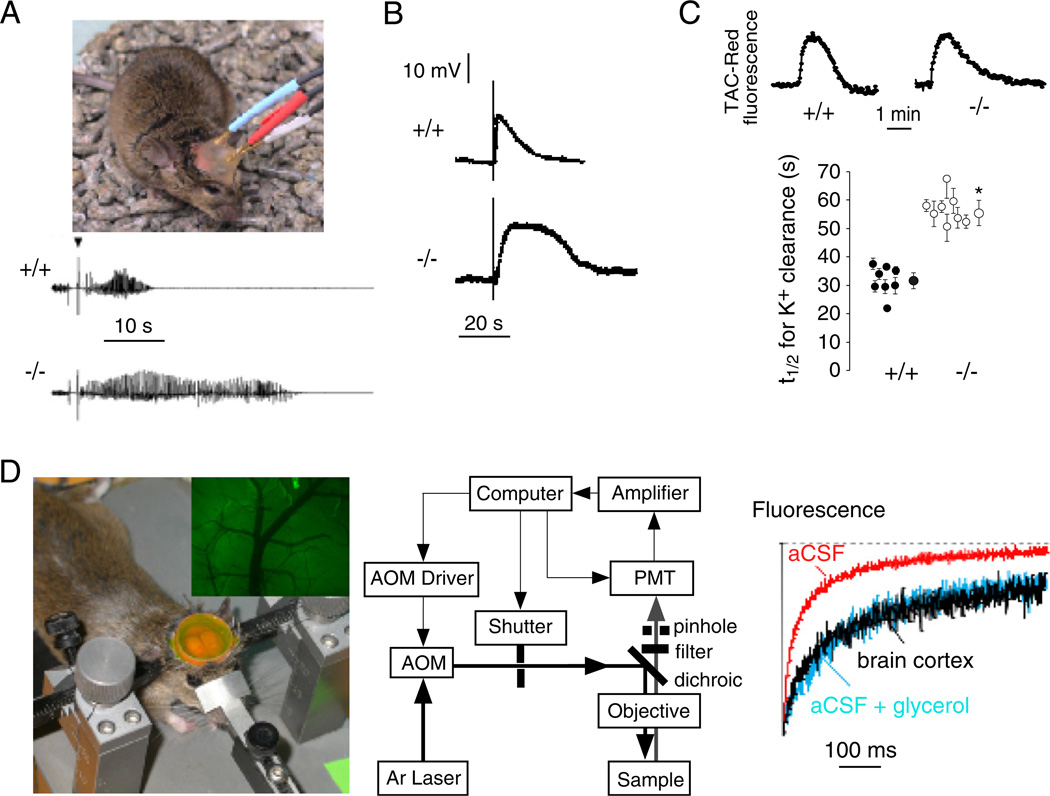
Phenotype analysis of AQP4 null mice has provided evidence for an unexpected role of AQP4 in neural signal transduction. AQP4 is expressed in supportive cells adjacent to electrically excitable cells, as in glia versus neurons in brain and spinal cord, Müller versus bipolar cells in retina and hair versus supportive cells in the inner ear. Based on this expression pattern, we found evidence for impaired auditory and visual signal transduction in AQP4 null mice, seen as increased auditory brainstem response thresholds (Li and Verkman, 2001) and reduced electroretinographic potentials (Li et al., 2002). In brain, seizure susceptibility in response to the convulsant (GABA antagonist) pentylenetetrazol was remarkably increased in AQP4 null mice (Binder et al., 2004a); at 40 mg/kg pentylenetetrazol, all wildtype mice exhibited seizure activity, whereas 6 out of 7 AQP4 null mice did not exhibit seizure activity. In a definitive study involving EEG recordings in freely moving mice (Fig. 4A, top), electrically induced seizures following hippocampal stimulation showed greater threshold and remarkably longer seizure duration in AQP4 null mice compared to wildtype mice (Fig. 4A, bottom) (Binder et al., 2006). In a related study, using a hyperthermia model of seizure induction, alpha syntrophin deficient mice (manifesting AQP4 mislocalization) developed more severe seizures than wildtype mice (Amiry-Moghaddam et al., 2003b).
Fig. 4.
AQP4 involvement in brain neuroexcitation. (A) Increased seizure duration in AQP4 null mice. Top. Bipolar electrodes implanted in the right hippocampus were connected to a stimulator and electroencephalograph recording system. Bottom. Representative electroencephalograms in freely moving mice following electrically induced generalized seizures. (B) Delayed K+ clearance in brain following electrically induced seizure-like neuroexcitation. Measurements done using K+-sensitive microelectrodes inserted into brain cortex in living mice. (C) Slowed K+ clearance in brain ECS during cortical spreading depression measured using TAC-Red, a K+-sensitive fluorescent probe. Top. Representative data. Bottom. Half-times (t1/2) for K+ reuptake. (D) Expanded brain ECS in AQP4 null mice measured by cortical surface photobleaching. Left. Mouse brain surface exposed to FITC-dextran with dura intact following craniectomy and fluorescence imaging of cortical surface after loading with FITC-dextran (inset). Middle. Photobleaching apparatus. A laser beam is modulated by an acousto-optic modulator and directed onto the surface of the cortex using a dichroic mirror and objective lens. Right. In vivo fluorescence recovery in cortex of wildtype mouse shown in comparison to aCSF and 30% glycerol in aCSF. Taken from Binder et al., 2006, Binder et al., 2004b and Padmawar et al., 2005. (See Color Plate 46.4 in color plate section.)
Several lines of evidence suggest delayed K+ uptake from brain ECS in AQP4 deficiency, which may account for the prolonged seizure phenotype. Direct measurements of K+ concentration in brain cortex in living mice using K+-sensitive microelectrodes showed significant slowing of K+ clearance following local stimulation by brief electrical pulses (Fig. 4B). Using a new triazacryptand-based K+-sensitive fluorescent dye, TAC-Red, altered K+ wave dynamics were found in a cortical spreading depression model of neuroexcitation, again with delayed K+ clearance (Fig. 4C). In related work, hippocampal slices from alpha syntrophin deficient mice showed slowed K+ clearance following evoked neuronal activity (Amiry-Moghaddam et al., 2003b). Impaired K+ clearance in AQP4 deficiency following neuroexcitation could account in part for the prolonged seizure activity in AQP4 deficiency, as well as altered evoked potential responses, but probably not for the reduced seizure susceptibility in AQP4 deficiency.
The molecular mechanism(s) responsible for impaired ECS K+ clearance in AQP4 deficiency remain unclear. It has been proposed that AQP4 is closely associated with the inwardly rectifying K+ channel Kir4.1. Immunocolocalization and immunopercipitation studies have suggested a close physical association of these two proteins in astroglia and Müller cells. However, recent patch-clamp studies at whole-cell and singlechannel levels in astroglia (Zhang and Verkman, 2008) and Müller cells (Ruiz-Ederra et al., 2007), provide direct evidence against functionally significant interactions between AQP4 and Kir4.1. Whether AQP4-facilitated water transport alone could account for the delay in ECS K+ clearance is unclear, as is whether AQP4 deficiency is associated with altered expression of other key proteins involved in ECS K+ handling.
We have obtained evidence for ECS expansion in AQP4 deficiency, which may account in part for reduced seizure susceptibility and prolonged seizure duration in AQP4 deficiency. An expanded ECS would provide a larger aqueous volume to dilute K+ released into the ECS during neuroexcitation, thereby slowing changes in ECS K+ concentration. Evidence for an expanded ECS in AQP4 deficiency came from cortical surface photobleaching measurements of the diffusion of fluorescently labelled macromolecules (Binder et al., 2004b). In this method the ECS in mouse brain was fluorescently stained by exposure of the intact dura to fluorescein–dextran after craniectomy (Fig. 4D, left). Fluorescein–dextran diffusion was detected by fluorescence recovery after laser-induced cortical photobleaching using confocal optics, as shown in Fig. 4D (middle). FITC–dextran diffusion was slowed ~ threefold in brain ECS relative to saline solutions (Fig. 4D, right). Diffusion of FITC–dextrans was significantly accelerated in AQP4 null mice, indicating an expanded ECS. ECS expansion in AQP4 deficiency was confirmed in follow-up studies utilizing a microfiberoptic epifluorescence photobleaching method to measure diffusion in deep brain structures (Zador et al., 2008). It remains unclear, however, whether ECS expansion in AQP4 deficiency could account quantitatively for the altered ECS K+ dynamics, as well as the mechanisms involved in chronic ECS expansion in AQP4 deficiency.
Indications and status of aquaporin modulators
Proposed indications of AQP4 modulators
Based on the above discussions, AQP4 modulators have therapeutic potential in several pathologies. The central role of AQP4 in controlling brain fluid balance suggests the utility of AQP4 modulators in the treatment of brain swelling. These drugs are expected to reduce brain water content and intracranial pressure, thus improving cerebral perfusion and preventing brain herniation and death. In this context, AQP4 modulators would be expected to act acutely and be administered for a few days whilst brain oedema remains a problem. AQP4 inhibitors or downregulators would be used to treat cytotoxic brain oedema caused by hypoxia, ischaemia or meningitis. AQP4 activators or upregulators would be useful in treating vasogenic brain oedema associated with brain tumour and brain abscess. Because the commonly used anti-oedema medications such as cortocosteroids do not modulate AQP4 function, AQP4 modulators used in combination with these drugs might have synergistic actions. AQP4 modulators may have side effects, however, potentially including auditory or visual disturbance. Such effects, if they occur, are expected to be transient and are clinically acceptable given the high morbidity and death associated with brain oedema. Many patients to be treated with AQP4 modulators will probably be comatose in Intensive Care Units, and therefore such side effects would be largely asymptomatic. AQP4 inhibitors might also be used to delay glial scar formation after brain injury to accelerate neuronal regeneration including axonal sprouting and synaptogenesis. In this context AQP4 inhibitors would be started a few days after the injury and administered for a few weeks, during the period of maximal glial scar formation. If AQP4 turns out to play an important role in the infiltration of astrocytomas, then AQP4 inhibitors might be used to treat these highly malignant tumours. By rendering astrocytomas less infiltrative with defined tumour–brain margins, AQP4 inhibitors may permit more complete surgical excision. Finally, AQP4 inhibitors may also be used to increase seizure threshold. Because their mechanism-of-action does not overlap with that of other anticonvulsants, AQP4 inhibitors might be used in combination with currently used antiepileptics.
Status and prospects for development of AQP4 inhibitors
There are at present no reported AQP inhibitors that are suitable candidates for clinical development. Though several AQPs are inhibited by sulfhydryl-reactive mercurials such as mercury and gold (Niemietz and Tyerman, 2002), these metal ions are non-specific in their action and toxic to living cells. Some candidate blockers of AQP1 have been reported, including tetraethylammonium (Brooks et al., 2000), acetazolamide (Ma et al., 2004) and DMSO (van Hoek et al., 1990); however, a careful evaluation of their inhibition efficacy using sensitive measurement methods indicated little or no AQP1 inhibition by tetraethylammonium or acetazolamide, and apparent inhibition by DMSO resulting from an osmotic clamp artefact rather than bona fide inhibition (Yang et al., 2006a). A recent paper reported strong AQP4 inhibition by multiple antiepileptics (Huber et al., 2007), though we were unable to verify their findings using appropriate assays (unpublished results).
The general approach for identification of new drugs, such as AQP inhibitors, is high-throughput screening in which large collections of diverse synthetic or natural small molecules is screened for their activity against a target (reviewed in Verkman, 2004). Active compounds are characterized for their potency, specificity and pharmacological properties, and optimized by testing chemical analogues. In addition to usual requirements such as good pharmacology, toxicity and stability properties, AQP4 inhibitors must penetrate into brain. Potential assays to identify AQP inhibitors have been developed, as discussed elsewhere (Verkman, 2001), and include an erythrocyte lysis assay that has been successful to identify nanomolar-potency inhibitors of a urea transporter (Levin et al., 2007). It remains to be established which assay(s) of AQP function are sufficiently robust for high-throughput drug screening, and whether AQP4 is a ‘druggable’ target suitable for discovery of high potency small-molecule inhibitors. Further, because AQP4 water transport function is probably already maximal, a search for small-molecule activators is unlikely to be successful; rather, transcriptional-level upregulators are likely needed to increased AQP4 water permeability.
Perspective
Phenotype analysis of AQP4 knockout mice has provided compelling rationale for the development of small-molecule AQP4 modulators as new therapeutic options for brain swelling, glial scarring and epilepsy. Though the identification and validation of a new drug target for brain disorders is potentially very exciting, particularly where therapeutic options are limited and often inadequate as is the case for brain swelling, there are significant challenges in implementing the idea of pharmacological AQP4 modulation. Discovery of potent and selective small-molecule AQP4 inhibitors is a major challenge, particularly with the requirement of efficient brain penetration. Of far greater challenge is the discovery of selective, small-molecule enhancers of AQP4 expression. AQP4 inhibitor therapy for brain swelling requires care because of complexities in cytotoxic versus vasogenic oedema. For example, AQP4 inhibition may be beneficial early in the course of stroke and head trauma, but of little utility or even deleterious later on. Similarly, AQP4 inhibition represents a two-edge sword in epilepsy therapy, as it increases seizure susceptibility as well as seizure severity. Notwithstanding these caveats, we consider the potential of AQP4 modulator therapy to be high and thus efforts focused on its implementation well justified.
References
- Amiry-Moghaddam M, Lindland H, Zelenin S, Roberg BA, Gundersen BB, Petersen P, Rinvik E, Torgner IA, Ottersen OP. Brain mitochondria contain aquaporin water channels: evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. Faseb J. 2005;19:1459–1467. doi: 10.1096/fj.04-3515com. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc. Natl. Acad. Sci. U.S.A. 2003a;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl. Acad. Sci. U.S.A. 2003b;100:13615–13620. doi: 10.1073/pnas.2336064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood–brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. Faseb J. 2004;18:542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- Aoki K, Uchihara T, Tsuchiya K, Nakamura A, Ikeda K, Wakayama Y. Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol. (Berl.) 2003;106:121–124. doi: 10.1007/s00401-003-0709-y. [DOI] [PubMed] [Google Scholar]
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. Faseb J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- Badaut J, Ashwal S, Tone B, Regli L, Tian HR, Obenaus A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr. Res. 2007;62:248–254. doi: 10.1203/PDR.0b013e3180db291b. [DOI] [PubMed] [Google Scholar]
- Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, Regli L. Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir. Suppl. 2003;86:495–498. doi: 10.1007/978-3-7091-0651-8_101. [DOI] [PubMed] [Google Scholar]
- Badaut J, Regli L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience. 2004;129:971–981. doi: 10.1016/j.neuroscience.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Binder DK, Oshio K, Ma T, Verkman AS, Manley GT. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport. 2004a;15:259–262. doi: 10.1097/00001756-200402090-00009. [DOI] [PubMed] [Google Scholar]
- Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J. Neurosci. 2004b;24:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J. Cereb. Blood Flow Metab. 2006;26:1527–1537. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005;95:254–262. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Regan JW, Yool AJ. Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol. Pharmacol. 2000;57:1021–1026. [PubMed] [Google Scholar]
- Chen CH, Xue R, Zhang J, Li X, Mori S, Bhardwaj A. Effect of osmotherapy with hypertonic saline on regional cerebral edema following experimental stroke: a study utilizing magnetic resonance imaging. Neurocrit. Care. 2007;7:92–100. doi: 10.1007/s12028-007-0033-9. [DOI] [PubMed] [Google Scholar]
- Dolman D, Drndarski S, Abbott NJ, Rattray M. Induction of aquaporin 1 but not aquaporin 4 messenger RNA in rat primary brain microvessel endothelial cells in culture. J. Neurochem. 2005;93:825–833. doi: 10.1111/j.1471-4159.2005.03111.x. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res. Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, Rash JE. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- Hu J, Verkman AS. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. Faseb J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007;17:1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Kastner R. Comparative studies on the astrocytic reaction in the lesioned central nervous system of different vertebrates. J. Hirnforsch. 1987;28:221–232. [PubMed] [Google Scholar]
- Ke C, Poon WS, Ng HK, Lai FM, Tang NL, Pang JC. Impact of experimental acute hyponatremia on severe traumatic brain injury in rats: influences on injuries, permeability of blood–brain barrier, ultrastructural features, and aquaporin-4 expression. Exp. Neurol. 2002;178:194–206. doi: 10.1006/exnr.2002.8037. [DOI] [PubMed] [Google Scholar]
- Kiening KL, van Landeghem FK, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF. Decreased hemispheric aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci. Lett. 2002;324:105–108. doi: 10.1016/s0304-3940(02)00180-5. [DOI] [PubMed] [Google Scholar]
- Klatzo I. Evolution of brain edema concepts. Acta Neurochir. Suppl. (Wien.) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- Levin MH, de la Fuente R, Verkman AS. Urearetics: a small molecule screen yields nanomolar potency inhibitors of urea transporter UT-B. Faseb J. 2007;21:551–563. doi: 10.1096/fj.06-6979com. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Ophthalmol. Vis. Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- Li J, Patil RV, Verkman AS. Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin-4 water channels. Invest. Ophthalmol. Vis. Sci. 2002;43:573–579. [PubMed] [Google Scholar]
- Li J, Verkman AS. Impaired hearing in mice lacking aquaporin-4 water channels. J. Biol. Chem. 2001;276:31233–31237. doi: 10.1074/jbc.M104368200. [DOI] [PubMed] [Google Scholar]
- Loitto VM, Huang C, Sigal YJ, Jacobson K. Filopodia are induced by aquaporin-9 expression. Exp. Cell Res. 2007;313:1295–1306. doi: 10.1016/j.yexcr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A. Aquaporin(s) expression in choroid plexus tumours. Pediatr. Neurosurg. 2006;42:228–233. doi: 10.1159/000092359. [DOI] [PubMed] [Google Scholar]
- Ma B, Xiang Y, Mu SM, Li T, Yu HM, Li XJ. Effects of acetazolamide and anordiol on osmotic water permeability in AQP1-cRNA injected Xenopus oocyte. Acta Pharmacol. Sin. 2004;25:90–97. [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Meng S, Qiao M, Lin L, Del Bigio MR, Tomanek B, Tuor UI. Correspondence of AQP4 expression and hypoxic-ischaemic brain oedema monitored by magnetic resonance imaging in the immature and juvenile rat. Eur. J. Neurosci. 2004;19:2261–2269. doi: 10.1111/j.0953-816X.2004.03315.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. Faseb J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat. Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. Faseb J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Archiv. 2008 doi: 10.1007/s00424-007-0357-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005;280:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007;22:778–784. doi: 10.1007/s00467-006-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Davidson KG, Yasumura T, Furman CS. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience. 2004;129:915–934. doi: 10.1016/j.neuroscience.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Mde C, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J. Neurosci. Res. 2006;83:1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- Rojek AM, Skowronski MT, Fuchtbauer EM, Fuchtbauer AC, Fenton RA, Agre P, Frokiaer J, Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Zhang H, Verkman AS. Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 potassium channels in retinal Muller cells. J. Biol. Chem. 2007;282:21866–21872. doi: 10.1074/jbc.M703236200. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg. Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005a;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell. Sci. 2005b;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, Brown D, Van Hoek AN. Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am. J. Physiol. Renal Physiol. 2004;287:F501–F511. doi: 10.1152/ajprenal.00439.2003. [DOI] [PubMed] [Google Scholar]
- Sun MC, Honey CR, Berk C, Wong NL, Tsui JK. Regulation of aquaporin-4 in a traumatic brain injury model in rats. J. Neurosurg. 2003;98:565–569. doi: 10.3171/jns.2003.98.3.0565. [DOI] [PubMed] [Google Scholar]
- van Hoek AN, de Jong MD, van Os CH. Effects of dimethylsulfoxide and mercurial sulfhydryl reagents on water and solute permeability of rat kidney brush border membranes. Biochim. Biophys. Acta. 1990;1030:203–210. doi: 10.1016/0005-2736(90)90296-z. [DOI] [PubMed] [Google Scholar]
- Verbavatz JM, Ma T, Gobin R, Verkman AS. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J. Cell Sci. 1997;110:2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Potential utility of aquaporin blockers as aquaretics. Drug News Perspect. 2001;14:412–420. doi: 10.1358/dnp.2001.14.7.858424. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Drug discovery in academia. Am. J. Physiol. Cell Physiol. 2004;286:C465–C474. doi: 10.1152/ajpcell.00397.2003. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, Cano J. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol. Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- Warth A, Simon P, Capper D, Goeppert B, Tabatabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood–brain barrier disturbance but not with patient survival. J. Neurosci. Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J. Biol. Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]
- Yang B, Kim JK, Verkman AS. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006a;580:6679–6684. doi: 10.1016/j.febslet.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zhao D, Verkman AS. Evidence against functionally significant aquaporin expression in mitochondria. J. Biol. Chem. 2006b;281:16202–16206. doi: 10.1074/jbc.M601864200. [DOI] [PubMed] [Google Scholar]
- Zador Z, Magzoub S, Jin S, Manley GT, Papadopoulos MC, Verkman AS. Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain. Faseb. J. 2008;22:870–879. doi: 10.1096/fj.07-9468com. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol. Cell. Neurosci. 2008;37:1–10. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]