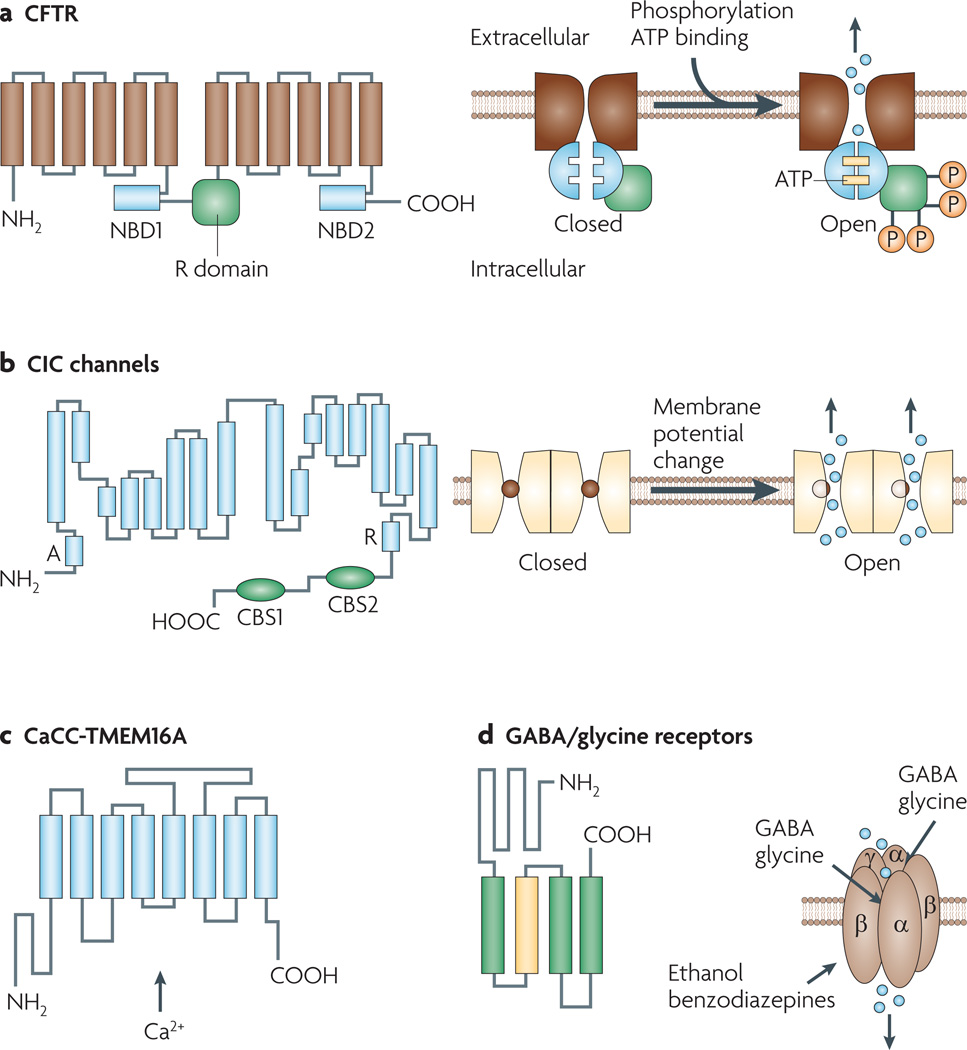
Figure 1. Structures and mechanisms of regulation of chloride channels.
a | Cystic fibrosis transmembrane conductance regulator (CFTR). Shown here are 12 membrane-spanning segments of CFTR plus two nucleotide binding domains (NBDs 1 and 2) and a regulatory R domain. CFTR activation involves cyclic AMP-dependent phosphorylation and binding of ATP molecules at the NBDs. b | The overall organization of voltage-gated chloride (ClC) channels is depicted, showing 18 segments (labelled A to R) most of which span the plasma membrane partially and in a strongly tilted configuration. Fast gating involves flipping of a pore-lining glutamate side chain into and out of the chloride pathway. Channels are arranged as dimers with a slow gate controlling the activity of both channels simultaneously. c | The calcium-activated chloride channel (CaCC) TMEM16A (anoctamin-1), with predicted topology showing eight transmembrane segments with cytosolic amino and carboxy termini. The mechanism of calcium activation is unknown. d | GABA (γ-aminobutyric acid) and glycine-gated chloride channels, showing pentameric channels formed by α, β and γ subunits. Each subunit has four transmembrane segments, with a large extracellular N terminus. The second transmembrane segment of each subunit contributes to the formation of the central pore. The N termini of the α and β subunits form the ligand binding site. Volume-sensitive chloride channels (not shown) have an unknown molecular structure. They activate upon cell swelling. CBS, cystathione β-synthase-related domain.