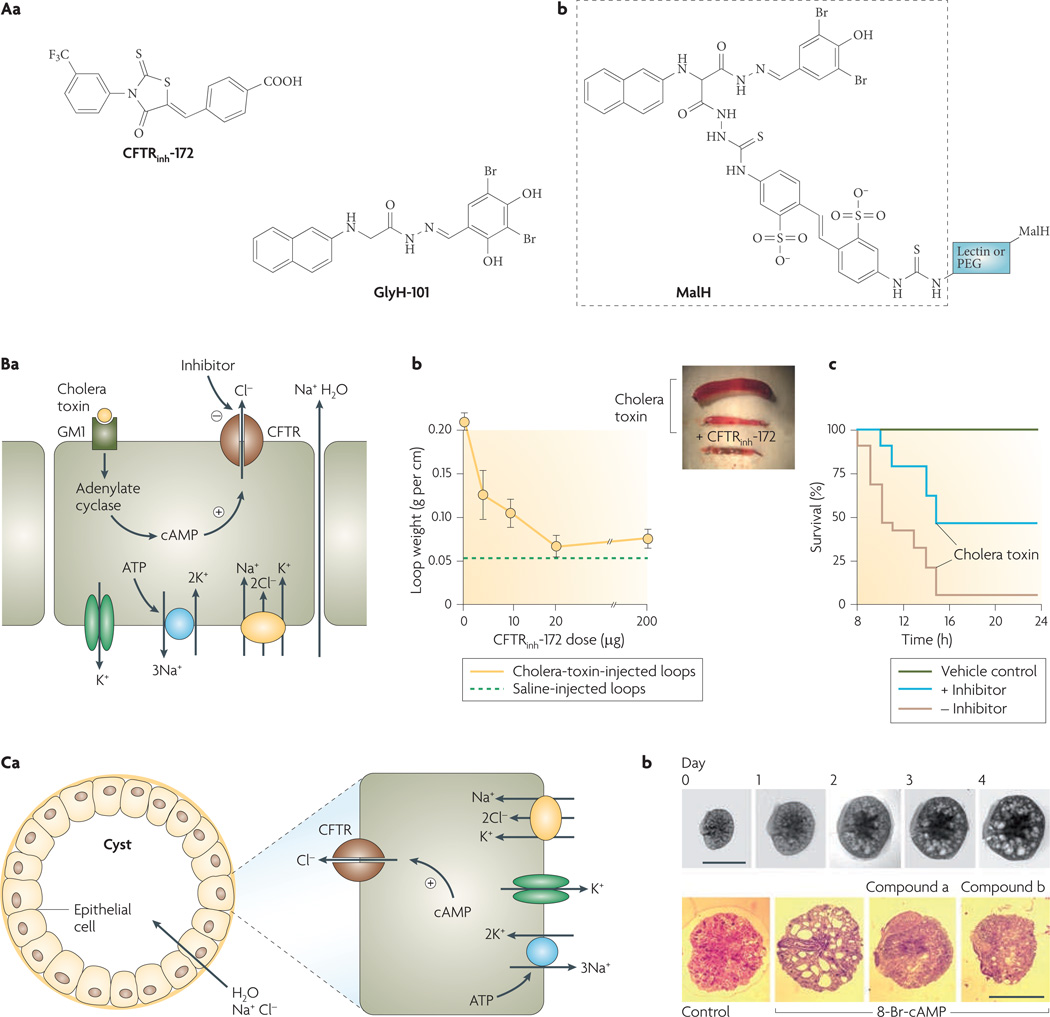
Figure 3. Cystic fibrosis transmembrane regulator (CFTR) inhibitors and their indications.
Aa | Structures of thiazolidinone CFTR inhibitor CFTRinh-172 and glycine hydrazide GlyH-101. Ab | Malonic acid hydrazide (MalH) conjugated to a macromolecular backbone (lectin or polyethylene glycol (PEG)). Ba | Intestinal fluid secretion in diarrhoea. Mechanism of enterotoxin-mediated diarrhoea showing CFTR chloride secretion following choleratoxin-induced cyclic AMP elevation. Sodium and water follow passively. Bb | CFTRinh-172 inhibits intestinal fluid accumulation in closed mouse ileal loops. Loops were injected with saline or cholera toxin and CFTRinh-172 was administered by intraperitoneal injection. Inset shows photograph of intestinal loops. Bc | Survival of suckling mice following oral administration of cholera toxin with or without CFTR inhibitor (MalH–lectin, 125 pmol). Vehicle control indicates no cholera toxin given. Ca | Cyst fluid secretion in polycystic kidney disease. Mechanism of fluid secretion into cysts, involving CFTR-dependent chloride secretion. Cb | CFTR inhibitors (compound a, a tetrazolo-derivatized thiazolidinone analogue; compound b, an absorbable, phenyl-derivatized glycine hydrazide analogue) cause slowing of cyst expansion in embryonic kidney organ culture. Upper panels show kidney growth and cyst formation in medium containing 8-Br-cAMP (scale bars, 1 mm). Bottom panels show day-4 kidneys in control and inhibitor-containing medium. Panel A is modified, with permission, from REF. 26 © (2007) W. B. Saunders. Panel B is modified, with permission, from REF. 28 © (2004) W. B. Saunders. Panel C is modified, with permission, from REF. 31 © (2008) American Society of Nephrology.