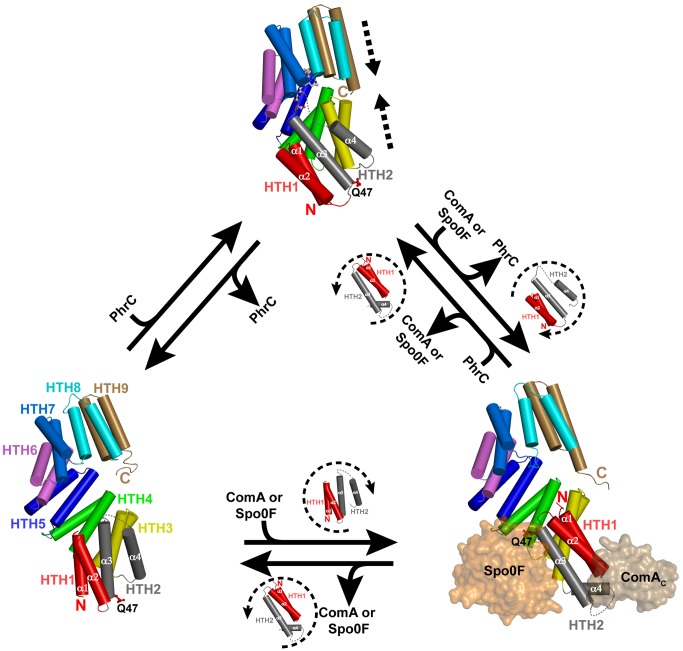
Figure 5. Rap protein conformations.
The structures depicted show a Rap protein alone (bottom left, RapI), Rap-target protein complexes [bottom right, RapH-Spo0F (PDB ID 3Q15) and RapF-ComAC (PDB ID 3ULQ)—for the sake of clarity RapF is omitted], and a Rap protein in complex with Phr peptide (top, RapJ-PhrC). The models surrounded by dashed arcs depict the movement of the Rap protein HTH1 and HTH2. In every panel, the sidechain of the catalytic glutamine corresponding to RapH Q47, RapJ Q47, and RapI Q53 is shown and labeled Q47. RapI alone (bottom left) is in an intermediate open conformation where HTH1 (red cylinders) and HTH2 (grey cylinders) extend the TPR domain (HTH3-HTH9) by two HTH repeats. RapH and RapF (bottom right) are in the fully open conformation, and they consist of an N-terminal 3-helix bundle (helices α1–α3), connected by the helix-α4 linker region, to a C-terminal TPR domain (HTH3-HTH9, colored as in the bottom left panel). RapJ-PhrC (top panel) is in the closed conformation. The direction of the Rap domain movements along the TPR domain superhelical axis are depicted by dashed arrows in the top panel.