Abstract
Cancer stem cells (CSCs), which comprise a small fraction of cancer cells, are believed to constitute the origin of most human tumors. Considerable effort has been focused on identifying CSCs in multiple tumor types and identifying genetic signatures that distinguish CSCs from normal tissue stem cells. Many studies also suggest that CSCs serve as the basis of metastases. Yet, experimental evidence that CSCs are the basis of disseminated metastases has lagged behind the conceptual construct of CSCs. Recent work, however, has demonstrated that CSCs may directly or indirectly contribute to the generation of metastasis. Moreover, CSC heterogeneity may be largely responsible for the considerable complexity and organ specificity of metastases. In this review, we discuss the role of CSCs in metastasis and their potential as therapeutic targets.
Keywords: Cancer stem cells, molecular marker, metastasis, epithelial to mesenchymal transition, circulating tumor cells, cancer therapeutic target
1. Introduction and cancer stem cells (CSCs)
The hypothesis that a small fraction of cancer cells, described as cancer stem cells (CSCs), are the basis of most tumors was first proposed in 1994. This work demonstrated that a small fraction of human leukemia cells could generate leukemia in severe combined immune deficient (SCID) mice, whereas the majority of tumor cells failed to engraft and establish disease (Lapidot et al., 1994). Further studies in hematopoietic malignancies suggested that CSCs, like normal stem cells (NSCs), reside at the apices of a hierarchical cluster of mature cells, possessing the ability to self-renew and generate multiple ‘mature’ progeny. Subsequent critical studies identified CSC activities in numerous solid tumors, including prostate (Collins et al., 2005a), colon (O'Brien et al., 2007; Ricci-Vitiani et al., 2007), head and neck (Prince et al., 2007), melanoma (Schatton et al., 2008), lung (Eramo et al., 2008), liver (Yang et al., 2008), breast (Al Hajj et al., 2003), brain (Singh et al., 2004), pancreas (Adikrisna et al., 2012; Li et al., 2007) (Hermann et al., 2007), ovary (Curley et al., 2009) and mesenchymal carcinomas (Wu et al., 2007).
Yet despite the convergence on the concept of CSCs, the definition and identification of these cells in most tumor types remains elusive and whether or not CSCs exist in all human tumors remains an open question. For example, the most striking example of a tumor that may not follow a stem cell model is melanoma. In this malignancy, a highly enriched CD271(+) population may have some CSC activity as demonstrated in one study showing CD271(+) cells able to develop tumors in Rag22/2cc2/2 mice while CD271 (-) cells did not (Boiko et al., 2010). However, other groups have shown that both the CD271(-) and CD271(+) cells obtained from patients with melanoma can generate tumors when implanted into non-obese diabetic (NOD)/SCID IL2Rγnull mice (Quintana et al., 2008; Quintana et al., 2010). Given these and other data, it has been proposed that melanomas follow a stochastic model rather than a hierarchical model of local tumor growth and distant spread (Quintana et al., 2008; Quintana et al., 2010).
In vivo studies, while central to the definition of what a CSC is, have fueled debate as to whether in vivo evidence of CSC characteristics requires specialized animal hosts with increasing immune deficiencies (Kelly et al., 2007; Nguyen et al., 2012). Yet, defining CSC activities based upon in vitro methods is also complex, and many groups have defined CSC activities based upon the ability to generate spheroids (Marsden et al., 2009; Suva et al., 2009). Others have suggested that the ability to exclude the dye Hoechst 33342 and/or the expression of the adenosine triphosphate-binding cassette (ABC) transporter ABCG2 characterizes a CSC (An & Ongkeko, 2009; Ding et al., 2010; Shien et al., 2012; Zenzmaier et al., 2008).
Elevated levels of aldehyde dehydrogenase (ALDH) (Storms et al., 1999) have also been proposed as a method to identify CSCs; however, ALDH does not appear to be a CSC marker in all tumor types (Yu et al., 2011a). Others have used a variety of cell surface markers to define CSCs from primary tumors and cell lines in a number of tumors, most notably CD133, CD44, CD24, and CD166 (Curley et al., 2009; Horst et al., 2009; Miki et al., 2007; Patel et al., 2010; Vermeulen et al., 2008; Zhang et al., 2012). Importantly, none of these studies has clearly defined CSCs as a single universal entity, suggesting that the CSC phenotype may vary substantially across different tumors.
There are a number of additional challenging aspects to the identification and characterization of CSCs. As mentioned previously, part of the controversy surrounding the CSC concept stems from the requirement that subpopulations of cell lines or cells from tumor samples form tumors in vivo. That differences in CSC activities can be traced to the model system employed should not be surprising, considering that differences in NSC activities have also been attributed to the animal hosts in which the test population is evaluated (Ito et al., 2012; Lepus et al., 2009; Notta et al., 2010). Each mouse model is likely to have differences in the expression of growth factors, cytokines/chemokines, adhesion molecules, and other environmental components which may be required for the successful establishment of xenotransplanted NSCs and CSCs (Kelly et al., 2007). Yet because of the diversity of responses in different model systems, defining universal characteristics of CSCs has proven illusive. In fact, attempts to define CSCs as a single entity or as a single developmental state may be a major source of the difficulty (Kelly et al., 2007; McCulloch & Till, 2005; Nguyen et al., 2012; Trosko, 2009).
In addition to the conceptual framework of CSCs, the origin of CSCs—and even the terminology pertaining to CSCs—remains controversial. There is evidence that some CSCs may be derived from NSCs. These may result from genomic mutations in NSCs which ultimately generate neoplastic growth (Massard et al., 2006). Other examples have demonstrated that when mesenchymal stem cells (MSCs) are inoculated into a breast cancer model, more metastases are observed, suggesting that transplantation of NSCs may promote tumorigenesis and metastasis and possibly the generation of CSCs (Karnoub et al., 2007). Likewise, neuronal stem/progenitor cells may participate in the generation of human glioma and provide the first example of a donor-derived brain tumor, suggesting that unregulated NSCs may produce tumors (Amariglio et al., 2009). Yet, it may not be true that CSCs are derived from NSCs that have undergone a multiple-hit oncogenesis (Nguyen et al., 2012). In fact, emerging data suggest that CSCs may be derived from progenitor, more ‘mature’ tissue cells (Hermann et al., 2007; Kelly et al., 2007; Li et al., 2010; McCulloch & Till, 2005; Nguyen et al., 2012; Trosko, 2009) or even cell fusions (Lu & Kang, 2009) (Figure 1). As a result, investigators have advocated for the term “tumor-initiating cells” to be utilized instead of CSCs, in order to avoid the implication that these cells are necessarily derived from NSCs (Neuzil et al., 2007).
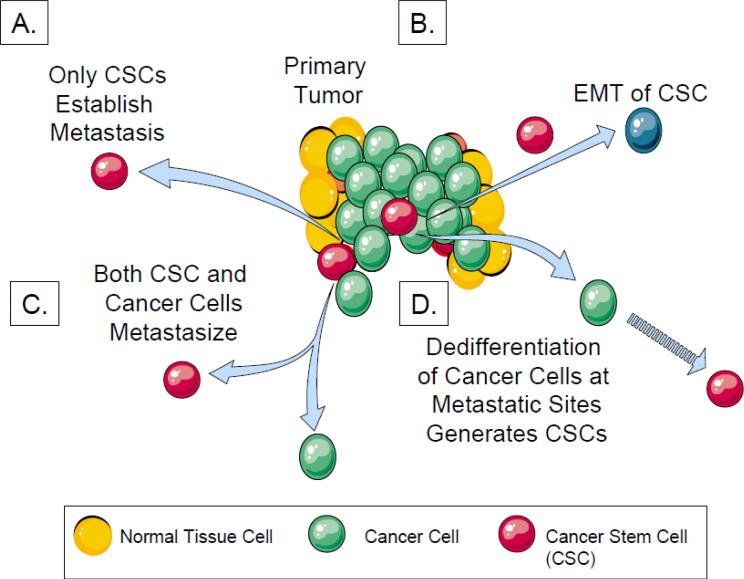
Fig 1. Multiple hypotheses examining the origin of CSCs and metastases.
A. Only the CSCs in primary tumors can home and metastasize to distant tissues directly.
B. CSCs are generated in primary tumors that ultimately undergo an EMT to generate CTCs. The CTCs home to distant tissues and undergo mesenchymal to epithelial transition establishing the disseminated CSCs.
C. In some specific tumor types, all the cancer cells including CSCs demonstrate no significant differences in the ability to generate tumors or establish metastases distant sites in animal models.
D. Non-stem-like cancer cells shed as CTCs from primary tumors circulate and home to distant tissues. Once in the distant site, the cancer cells became CSCs through de-differentiation.
One of the key underlying hypotheses of the CSC model proposes that CSCs are the basis of metastases. Yet, experimental evidence that CSCs produce disseminated metastases has lagged behind the conceptual construct of CSCs. In this review, we will discuss evidence supporting the role of CSCs in metastasis, their characteristics in the metastatic niche, and their potential as therapeutic targets in order to prevent the development of clinically overt metastatic disease.
2. Molecular features of CSCs that are linked to metastasis
Genetic signatures in CSCs are thought to predict tumor recurrence and metastases, providing some support for the concept that CSCs may be metastatic precursors (Fig1). For example, expression of the CSC marker CD133 in glioblastoma and lung adenocarcinoma is correlated with both the proliferation marker Ki67 and poorer clinical outcomes (Pallini et al., 2008; Woo et al., 2010). CD133 antigen expression has also been shown to correlate with patient survival in high-grade oligodendroglial tumors (Beier et al., 2008), rectal cancer (Wang et al., 2009), gastric adenocarcinoma (Zhao et al., 2010), and non-small cell lung cancer(Shien et al., 2012). Additionally, in patients with colorectal carcinoma, the combination of CD133, CD44, and CD166 can successfully identify patients at low-, intermediate-, and high-risk of recurrence and metastasis (Horst et al., 2009). Likewise, methylation of Wnt-target-gene promoters are also strong predictors for recurrence in colorectal cancer, suggesting that CSC gene signatures, rather than reflecting CSC numbers, may reflect differentiation status of the malignant tissue and the risk for dissemination (de Sousa et al., 2011).
One of the key steps in the metastatic cascade is the migration of tumor cells away from the primary tumor, and CSCs likely facilitate this migration. The ability of cells to migrate from one location to another is a critical normal process in development, and tumor cells appear to capitalize on the underlying physiologic mechanisms. Most adult tissues maintain some aspect of this migratory capacity through the ability to generate an epithelial to mesenchymal transition (EMT)-like process during wound healing, tissue regeneration and organ fibrosis. It has been hypothesized that CSCs may also activate their migration through the process of EMT (Figure 1). In fact, the mesenchymal phenotype marker Zeb1 may facilitate the acquisition of stem cell-like properties (Peter, 2010). Similarly, untransformed immortalized human mammary epithelial cells are capable of undergoing an EMT-like state by expressing FoxC2, Zeb factors, and N-cadherin, all of which have been linked to a CSC state (Morel et al., 2008). Likewise, by over-expressing Ras or Her2/neu, a stem-like subpopulation of CD44high/CD24low cells with an enhanced EMT phenotype has been identified (Radisky & LaBarge, 2008). It is possible that the EMT process is a critical aspect of CSCs and may not be entirely dependent on outside signals as data suggest that tumor stroma which promote metastasis, may not induce a EMT in tumor cells (Karnoub et al., 2007). This suggests that the EMT process occurs independently in carcinoma cells. On the other hand, the acquisition of an EMT may not be solely a cell-autonomous feature of cancer cells but may be regulated by signals from the microenvironment or niche. Along these lines, tumor- or cancer- associated fibroblasts have been shown to enhance the metastatic potential of tumors by promoting migration and extravasation through an EMT process as well as the establishment of a CSC-like state (Aktas et al., 2009; Armstrong et al., 2011; Gregory et al., 2008; Kalikin et al., 2003; Martin et al., 2010; van der, 2011).
Other factors are also thought to participate in the establishment of a metastatic phenotype by CSCs. During the final stages of cell division, each daughter cell must loose contact with each other to generate independent progeny. The final step in this process occurs within a tube or bridge that connecting the daughter cells. A protein structure called midbody is essential for the process of separating the two cells. Cancer cells accumulate midbody derivatives which enhance the tumorigenicity of cancer cells (Kuo et al., 2011). Moreover, several microRNAs (miRNAs) may also participate in the activation of CSC-like activities, where loss of miR-124, which regulates proliferation, has been shown to promote glioma formation (Cheung & Miller, 2001). KLF4, which is used extensively to ‘reprogram’ somatic cells into stem-like states, has potent oncogenic activities in mammary tumorigenesis and is likely involved in maintaining stem cell-like features in tumor cells (Yu et al., 2011b). Expression of phosphatase and tensin homolog (PTEN) is also thought to be critical for the establishment of a stem-like state (Zhou et al., 2007), and its loss correlates with prostate cancer metastasis and progression. Recent studies linking the RAS/MAPK pathway with PTEN deletion demonstrated the development of an EMT state in the prostates of experimental animals with 100% penetrance of metastasis (Mulholland et al., 2012). Here, a novel stem/progenitor subpopulation of EpCAMlow/CD24low cells with mesenchymal characteristics was found to regenerate the primary lesions upon orthotopic transplantation and to be highly metastatic (Mulholland et al., 2012).
3. Circulating Tumor Cells (CTCs) and CSCs
A number of studies have linked circulating tumor cells (CTCs) to tumor progression in a variety of solid tumors, and CTC enumeration has begun to be utilized as a prognostic tool in patients with metastatic breast (Cristofanilli et al., 2004), colon (Cohen et al., 2008) and prostate cancer (Danila et al., 2007). However, whether CTCs are simply associated with disease worsening or whether they directly contribute to metastatic progression remains to be determined. Potentially, a fraction of CTCs have CSC activity, and it is hypothesized that CSCs in a primary tumor which enter the circulation become circulating CSCs and remain so until they lodge or home to a target organ. If true, then stem-like CTCs may be a critical subset of CTCs with the capacity to form distant metastases. Recently, CTCs isolated from patients with melanomas have been found to generate metastases in xenotransplantation models (Ma et al., 2010). Further characterization of these cells may offer critical insight into the mechanisms underlying tumor spread and the contribution of CSCs.
For example, if indeed the spread of CSCs leads to metastasis then it would be expected that some CTCs would express stem cell markers (Aktas et al., 2009; Kasimir-Bauer et al., 2012). So far, defining the CSCs in a population of CTCs has proven extremely challenging given current limitations in the capture of CTCs (Monteiro & Fodde, 2010). Nevertheless, a recent study demonstrated that 66.7% of breast cancer patients demonstrate a putative stem cell/progenitor phenotype (35.2% CD44 (+)/CD24 (-/low) or 17.7% ALDH1 (high)/CD24 (-/low)) in CTCs (Theodoropoulos et al., 2010). A second line of evidence has shown that CTCs obtained from patients with Dukes’ B and C colon cancers express CD133, carcinoembryonic antigen (CEA) and cytokeratin. Prognosis among these patients is significantly poorer due to metastasis than those individuals who were found not to express these markers in their CTCs (Hou et al., 2012; Pantel & Alix-Panabieres, 2007).
4. CSCs and the metastatic niche
Similar to NSCs, CSCs are thought to reside in a relative stable microenvironment, or niche, in order to retain an undifferentiated state and give rise to more differentiated progenitor cells (Calabrese et al., 2007). From the perspective of metastasis, it has been reported that VEGFR1 expressing marrow cells participate in the process by establishing “pre-metastatic niches” (Kaplan et al., 2005b).
Observations into the earliest steps of metastasis preceding the influx of tumor cells into distant organs form the basis for the pre-metastatic niche hypothesis. Flow cytometry and immunofluorescence studies coupled with cells labeled with green fluorescent protein (GFP), demonstrate that cell clusters of bone marrow derived cells in the parenchyma of metastatic organs precede the arrival of fluorescently labeled tumor cells. It has been gleaned that the bone marrow-derived cells express VEGFR1 and several other hematopoietic markers including CD34, CD11b, c-kit, and Sca-1, defining the cells as early hematopoietic progenitors cells engaged with the parenchyma of the distant organ (Kaplan et al., 2005b; Kaplan et al., 2006; Kaplan et al., 2007; Wels et al., 2008). The progenitors also express the VLA-4 (α4β1), thus priming them to sites rich in fibronectin to establish the clusters in preparation for metastasis (Kaplan et al., 2005a; Kaplan et al., 2005b). It is thought that these cells may become educated within tumors to ultimately hunt out and lay the foundations for distant metastasis. Alternatively, the cells may become activated locally or in the circulation due to chemokines secreted by tumor. One molecule which has been implicated in this regard is placental growth factor (PlGF), a ligand for VEGFR1. PlGF secretion may activate resident organ fibroblasts to synthesize fibronectin, which facilitates the binding of VLA-4 expressing hematopoietic progenitor cells (Peinado et al., 2012). Exosomes are another class of tumor derived products which may prime metastases. Exosomes from highly metastatic melanomas have been demonstrated to increase the establishment of metastases by ‘educating’ marrow hematopoietic progenitors to express the receptor tyrosine kinase MET (Peinado et al., 2012) . Consequently, activation of MET by hepatocyte growth factor (HGF) renders the marrow cells more migratory and capable of establishing pre-metastatic niches (Peinado et al., 2012). Similar results have been observed following the release of microvesicles from human renal CSCs which stimulate angiogensis and the formation of pre-metastatic niches in lungs (Atala, 2012; Grange et al., 2011). In addition to directly activating the bone marrow hematopoietic components, melanoma-derived exosomes also induce leakiness of the vasculature are pre-metastatic sites setting in motion the arrival of marrow progenitors (Peinado et al., 2012).
Yet, the work which most directly implicates CSCs as metastatic seeds in conjunction with a metastatic niche is work on tenacin C (TNC) expression in CSCs. It appears that the ability to express TNC is a central freature of the ability of CSCs to establish metastasis in the lungs of experimental animals (Oskarsson et al., 2011). The cancer-derived TNC enhances the expression of musashi homolog 1 (MSI1) and leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) (Oskarsson et al., 2011), both of which have stem cell modulating activities. MSI1 regulates NOTCH signaling, and LGR5 is a target of the WNT pathway.
Each of these pathways have been shown to be critical for the metastasis and in stem cell biology (de Sousa et al., 2011; Duncan et al., 2005; Malanchi et al., 2012; Reya & Clevers, 2005) . It is however important to note that TNC did not altered the antigen profile of breast CSCs, suggesting that CSC activities were established prior to metastasis, and TNC is may be critical for maintenance of stem cell functions (Oskarsson et al., 2011).
5. CSCs as niche parasites
Stephen Paget's observation that tumor “seeds” seek out “fertile soil” represents a well-known paradigm for why certain cancers localize to preferred sites with a high propensity (Paget, 1889). The bone marrow microenvironment has been well established as a regulatory site for hematopoietic function. In the marrow, hematopoietic stem cells (HSCs) are believed to localize to the specific microenvironment, or the niches, to engage in a quiescent state to preserve their self-renewal capacity, or divide and differentiate to populate their corresponding lineages (Haylock & Nilsson, 2005; Taichman, 2005; Zhu & Emerson, 2004). Two such niches have been described: a vascular niche and an endosteal niche. The majority of HSCs as detected by immunohistochemistry appear to be in the vascular niche, richly compromised of endothelial cells. In the vascular niche, it is thought that HSCs are engaged in active surveillance of hematopoietic need, facilitating a rapid response to hematopoietic demands (Kopp et al., 1920; Li & Li, 2006). HSCs in the endosteal niche are thought to be more quiescent or represent a reserve population (Calvi et al., 2003; Nishikawa et al., 2008; Patt et al., 1980; Zhang et al., 2003). The principal regulatory cells of the endosteal HSC niche are thought to be osteoblasts, adipocyctes, mesenchymal stem cells and CXCL12-abundant reticular or CAR cells (Shiozawa et al., 2008b; Sugiyama et al., 2006; Taichman et al., 1996). Osteoblasts appear to be major regulators of endosteal niche function, as they have been shown to express a number of cytokines, growth factors, and adhesion molecules critical to HSC regulation.
Parallel to the concept of the HSC niche, growing evidence have suggested that disseminated tumor cell (DTCs) also localize to and reside in the bone marrow niche (Shiozawa et al., 2008b). In fact, prostate cancer DTCs use similar mechanisms as HSCs in order to gain access to the niche. This includes expression of the stromal derived factor-1 (SDF-1 or CXCL12) pathway as well as adhesion molecules utilized by HSCs, facilitating successful survival of these cells within the marrow microenvironment (Shiozawa et al., 2008a; Sun et al., 2005; Sun et al., 2008; Taichman RS et al., 2002) (Figure 2). Further work from our group has recently demonstrated that DTCs target and displace HSCs out of their niche, and establish metastatic foci within the niche space (Shiozawa et al., 2011a) (Figure 2). This was demonstrated using an in vivo micrometasasis model in which DTCs were introduced into immunedeficient mice and permitted to take up residence in the bone marrow (Havens et al., 2008). Thereafter, the mice were given bone marrow transplantations to determine the ability of HSCs to engraft into their marrow niches (Shiozawa et al., 2011a). Significantly fewer HSCs engrafted into the mice harboring tumor cells in their marrow, suggesting that the tumor cells were occupying these niches and preventing HSC localization and engraftment. Importantly, a competitive engraftment was performed to determine if DTCs directly out-compete HSCs for niche space (Shiozawa et al., 2011a). In this experiment, existing niches were cleared by lethally irradiating mice. HSCs were given to all mice via transplant to rescue the mice from the lethal irradiation, and in some groups (prostate cancer) PCa cells were also implanted. Mice with tumors cells showed markedly less engraftment and thus decreased survival, indicating that PCa cells out-competed HSCs for their niches (Shiozawa et al., 2011a). These findings have lead to the concept of DTCs as “parasites” of the HSC niche (Shiozawa et al., 2011b; Shiozawa et al., 2011c).
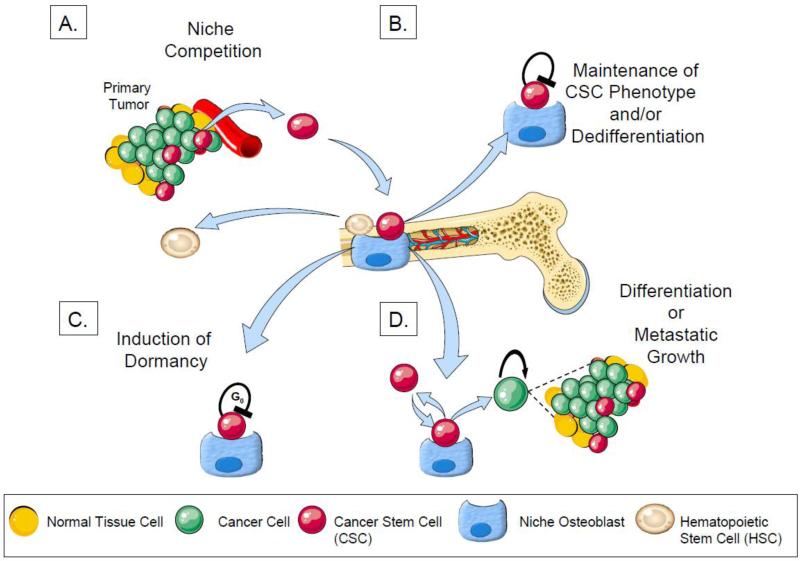
Fig 2. Competition and engagement of the HSC niche and its role in CSC maintenance and dormancy.
A. CSCs compete with HSCs for engagement of the osteoblastic HSC niche which regulates quiescence and proliferation of HSCs. HSCs which are displaced from the niche are found in the peripheral circulation.
B. PCa cells which enter the niche as either CSCs are or more mature cells are “dedifferentiated”into CSCs and or maintained as stem cells.
C. Once in the HSC niche, CSCs may undergo either growth arrest or exit the cell cycle in G0 and become dormant
D. After asymmetric cell division, progeny of CSCs may either return to the niche as a CSCs or may terminally differentiate following additional proliferative activities which result in the establishment of metastatic bone lesions.
One of the central questions that arises from these studies is whether stem-like characteristics of some of these DTCs explains the phenomenon of these cells targeting the bone marrow niche. Evidence again from our group has shown that when DTCs engage the niche they become quiescent. We demonstrate that the binding of PCa to annexin II induces the expression of the growth arrest specific-6 (GAS6) receptors AXL, Sky, and Mer which in the hematopoietic system induce dormancy (Dormady et al., 2000). In addition, GAS6 produced by osteoblasts prevents PCa proliferation and protects PCa from chemotherapy-induced apoptosis. Our results suggest that the activation of GAS6 receptors on PCa cells in the bone marrow environment may play a critical role as a molecular switch establishing metastatic tumor cell dormancy.
Additionally, this hypothesis may elucidate critical aspects of tumor tropism, a still unexplained phenomenon observed in all malignancies. Using in vivo murine models of human PCa cell metastasis to bone, it has been noted that the majority of skeletal metastasis are located in either the spine or the hindlimbs ((Jung et al., 2012b). Much less prevalent are lesions found in the bones of the forelimb, and normalizing tumor prevalence data to account for the number of PCa cells arriving following intravascular injection, marrow cellularity and number of hematopoietic stem cell (HSC) niches does not explain this difference. However, an analysis of differential gene and protein levels lead us to identify GAS6 expression as significantly greater in the forelimb compared to the hindlimb bone marrow (Jung et al., 2012a). When murine RM1 prostate cancer cells were implanted into s.c. spaces in immune competent animals, tumor growth in the GAS6-/- animals was greater than in GAS6+/+ animals. In an osseous environment, the human PC3 cell line grew significantly better in vertebral body transplants (vossicles) derived from GAS6-/- animals than in vossicles derived from GAS6 wild-type animals. These data suggest that the differences in tumor prevalence following intravascular inoculation may be a useful model to study the molecular basis of tumor dormancy (Jung et al., 2012a). Combined, our work on the niche suggests,that the ability to establish dormancy may be a central feature of CSCs. If true, then perhaps the ability of the niche to establish dormancy could be used as a way to define CSCs.
The CSC hypothesis suggests that most carcinomas are organized in a hierarchical pattern in which CSCs generate a population of non-renewing cells that form the bulk of the tumor. An alternative hypothesis proposes that non-stem cell-like CTCs are induced to generate CSC-like cells in the new microenvironment. This is supported by several reports suggesting that the niche can induce non-tumorigenic cells to assume a CSC-like phenotype (de Sousa E Melo et al., 2011; de Sousa et al., 2011; Vermeulen et al., 2008). For example, HGF-producing myofibroblasts are able to alter non-tumorigenic cells into tumor propagating cells by reactivating the Wnt pathway. These “dedifferentiated” cancer cells display all the characteristics of CSCs, including expression of stem cell–associated genes (e.g. LGR5), and have high tumorigenic potential (de Sousa E Melo et al., 2011; de Sousa et al., 2011; Vermeulen et al., 2008). These and other data suggest that NSCand CSC-like cells can arise de novo as well as from more differentiated cell types following expression of transcription factors such as Oct4, Sox2, c-Myc, Nanog and KLF4(Ben Porath et al., 2008; Chiou et al., 2010; Collins et al., 2005b; King et al., 2011; Li et al., 2011; Miyoshi et al., 2010; Takahashi & Yamanaka, 2006). Thus, the hierarchical model of stem cells should take into account bidirectional conversions between both stem and non-stem cell compartments (Reviewed in Li (Li & Laterra, 2012)).
6. CSCs and metastasis
While the hypothesis that CSCs directly contribute to the initiation of metastases has gained increasing acceptance, most of the supporting evidence comes from studies demonstrating associations between CSC markers and cellular phenotype. Examples include reports demonstrating that CD44 expression by breast(Al Hajj et al., 2003) and pancreatic(Hermann et al., 2007) tumor cells have enhanced metastatic capabilities. Similarly, expression of the chemokine receptor CXCR4 by breast (Muller et al., 2001) and prostate cancers (Taichman RS et al., 2002) have been shown to impart critical stem cell functions on these cells (Kucia et al., 2005; Lee et al., 2012; Rhim et al., 2011). Importantly, however, the expression of markers such as CD44 or CD24 -/low by tumor cells alone does not prove that these cells can generate metastases—or even that they are necessarily CSCs. Similarly, identification of CSCs within metastatic lesions or in circulating or disseminated tumor cell populations (Balic et al., 2006) does not necessarily mean these cells are capable of establishing disseminated lesions. There remains no direct experimental evidence that CSCs shed from a primary lesion can generate tumors at distant sites. The corollary also remains unproven—that targeted blockage of CSCs in the blood or lymphatic systems can prevent metastases from forming. Demonstrating these proposed chracteristics of CSCs has, to date, proven extremely difficult due to the insensitivity of current metastatic. Additionally, it is possible that several CSC phenotypes may coexist within tumors, each with unique chemosensitivities and metastatic potential, and therefore a full understanding of CSC characteristics within a given tumor remains elusive (Kang et al., 2003; Minn et al., 2005).
Some of the most direct evidence that CSCs establish metastases comes from the demonstration that breast cancer CSCs isolated based upon the putitive stem cell markers CD44+ and CD24-/low, are able to generate primary tumors in an orthotopic site and subsequently produce lung metastases (Liu et al., 2010). Likewise, orthotopic implantation of pancreatic cancer cells expressing a CSC phenotype generate distant liver metastases (Hermann et al., 2007). Interestingly, different populations of CSCs may be responsible for primary vs. secondary tumor sites, implicating CSC heterogeneity as a critical component of this model. Recent work by Dieter et. al. has demonstrated this heterogeneity within the CSC compartments, reporting at least three phenotypically distinct CSCs in a human colon cancer animal model (Dieter et al., 2011). To track the contribution of tumor-initiating cell clones, the group generated tumorigenic cells from cancer specimens, marked them with lentiviral vectors, and then sequenced the integration sites in serially transplanted tumors. A population of CSCs was identified as tumor transient amplifying cells (T-TACs) which had limited self-renewal capacity but did form tumors in primary transplants. A second population of CSCs exhibiting extensive self-renewing long-term tumor initiating cells (LT-TICs) were able to generate tumors in serial xenotransplants. A third population descried as rare delayed contributing TICs (DC-TICs) were exclusively active in secondary or tertiary mice. Interestingly, the marrow could serve as an major source of LT-TICs, however metastasis formation was predominantly driven by self-renewing LT-TICs (Dieter et al., 2011). This study therefore provides an exquisite evaluation of the clonal contribution to metastasis and tumor formation from individual CSC clones. The study also confirms the heterogeneity among CSCs in colon cancers and provides perhaps the most direct evidence to date that CSCs may contribute to metastasis (Dieter et al., 2011).
7. Therapeutic targeting of metastatic CSCs
The CSC hypothesis has fundamental and profound implications for cancer therapies, as CSCs are believed to be more resistant to chemotherapy than more differentiated cancer cells. To directly target CSCs, it will be critical to identify novel and unique pathways that are active in this subset of tumor cells. One example of this is the use of an activating monoclonal antibody directed at CD44,,which resulted in significant reductions in the levels of acute myeloid leukemia in one in vivo study (Jin et al., 2006). Importantly, CD44 is a regulator of several miRNAs known to maintain CSCs. In fact, when CD44 expression in PCa cells is down-regulated, miR-34a levels increase leading to reduced tumor regeneration and metastasis in xenografts (Liu et al., 2011). Similarly targeting of salinomycin in breast cancer inhibits tumor growth, induces epithelial differentiation of tumor cells, and down-regulates CSC genes in tumor cells (Gupta et al., 2009). However, whether these strategies will work in other tumor types remains to be determined, and how they can be used to limit or irradicate metastasis is unknown.
The ability of CSCs to expand in a rapid fashion after an exaggerated period of quiescence is thought to represent one of the most daunting challenges for the development of therapeutic interventions in cancer, particularly in the metastatic setting. Combination therapies therefore are likely to be required to target both proliferating cells and CSCs and will likely integral to this approach. Recently, two interesting studies provide promising evidence for the combination of CSC targeting therapy and conventional chemotherapy. The first study demonstrated that a neutralizing antibody against a membrane-associated NOTCH ligand inhibits tumor growth and CSC self-renewal in human colon cancer implanted mice (Hoey et al., 2009) . Likewise, the second study revealed that the administration of an anti-CD123 antibody prevents the engraftment of serially transplanted acute myeloid leukemia into the animals, suggesting this antibody impedes the stem-like characteristics of leukemia cells (Jin et al., 2009). In both cases, the inhibitory abilities of the antibodies were enhanced when animals were treated in combination with chemotherapy (Hoey et al., 2009; Jin et al., 2009) (Figure 3) .
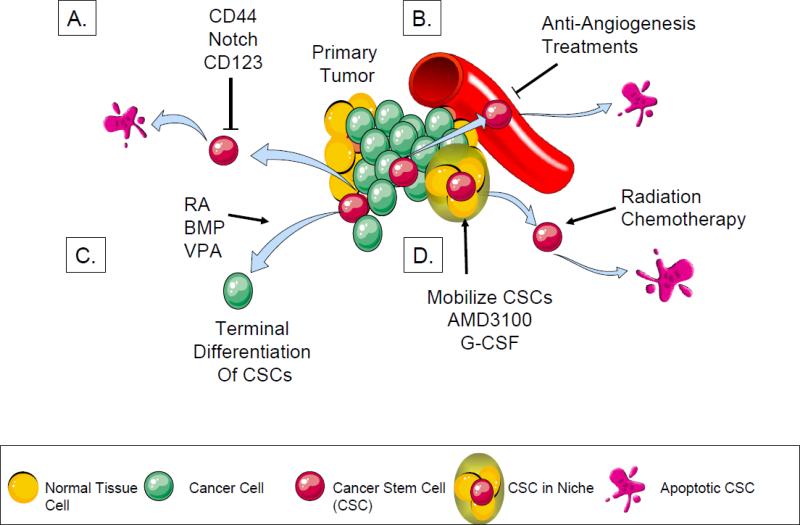
Fig 3. Potential therapeutic strategies to specifically target metastatic CSCs.
E. CSCs are eradicated with compounds that selectively target the CSC phenotype.
F. CSCs spontaneously undergo apoptosis when the nutritional support from the surrounding vasculature is withdrawn.
G. CSCs can be terminally differentiated to avoid proliferation and dedifferentiation.
H. Once CSCs reside within the niche, they are thought to become dormant. If CSCs are displaced from the niche, they are activated and therefore become susceptible to cytotoxic treatment.
Another proposed therapeutic approach is to stimulate differentiation of CSCs such that they lose their capacity for self-renewal and resistance to chemotherapeutic agents (Nguyen et al., 2012) (Figure 3). This is particularly critical where CSCs are widely distributed at low density, making conventional interventions challenging. Thus far, the most well developed therapeutic agent is vitamin A and its analogues (retinoid acid) for the treatment of acute promyelocytic leukemia (APL). These agents enhance tumor differentiation and reverse malignant progression by modulation of signal transduction networks regulated by nuclear retinoid receptors. In patients with APL, a 90% remission rate and a 70% cure rate with all-trans-retinoic acid therapy followed by chemotherapy has been observed (Burnett et al., 2010). In vitro, retinoid acid can also induce differentiation in embryonic cells, teratocarcinomas and melanomas (Rohwedel et al., 1999).
As stem cells are often dependent on bone morphogenetic protein (BMP) signaling, a number of therapeutic strategies are being sought to target this pathway (Joseph et al., 2012; Zhang et al., 2003; Zhu & Emerson, 2004). Piccirillo et al reported that the BMP-BMPR signaling system - which controls the activity of normal brain stem cells - may also act as a key inhibitory regulator of tumor-initiating, stem-like cells from glioblastoma by a reduction in proliferation and increased expression of markers of neural differentiation (Piccirillo et al., 2006). The reduction in the size of the CD133(+) population and the growth kinetics of the glioblastoma cells suggest that targeted BMP-pathway therapeutics are worth pursuing (Massard et al., 2006; Piccirillo et al., 2006) (Figure 3). In addition, the histone deacetylase inhibitor valproic acid, commonly used to treat epilepsy, has recently been found to have anti-cancer activity that may target CSCs (Blaheta et al., 2005) . Valproic acid induces the terminal differentiation of cancer cells by increasing the DNA binding of activating protein-1 transcription factor, decreasing protein kinase C (PKC) activity, inhibiting the WNT signaling pathways, and activating the peroxisome proliferator-activated receptors, in addition to blocking histone deacetylase (Blaheta et al., 2005). Although further study is clearly needed, these classes of agents may be useful for reducing CSC activities both in the local setting and in the context of disseminated CSCs.
However, the challenges of targeting disseminated CSCs may be even more pronounced, as the distant microenvironment may help protect these cells from therapeutic insults(Hovinga et al., 2010). In particular, the vasculature likely plays an important role in forming stem and progenitor cell niches and has been suggested to regulate many tumor microenvironments (Bautch, 2011). Therefore, damaging the CSC niche environment may impact the survival and tumor-initiating properties of CSCs (Folkins et al., 2007). The impact of angiogenesis inhibitors such as bevacizumab, thalidomide, sorafenib, sunitinib, pazopanib may be in part related to their effects on the vascular niche and disruption of the CSC microenvironment (Tonini et al., 2003).
Other niche regulating targets are also emerging. Periostin is a major component of the extracellular matrix, expressed by fibroblasts in the normal tissue and in the stroma of many tumors. Recent work has shown that the ability of infiltrating tumor cells to induce stromal production of periostin at secondary target organs impacts colonization and stem cell maintenance. Importantly, blocking periostin function may prevent metastasis by preventing the recruitment and activation of the WNT signaling in CSCs (Malanchi et al., 2012).
A second and interesting aspect of therapies designed to target the microenvironment is the possibilities that CSCs may be released from the microenvironment or niche (Figure 3). If CSCs utilize the niche to maintain their stemness, become dormant and therefore acquire the chemoresistance, this therapeutic strategy could enhance the susceptibility of CSCs to chemotherapy. Indeed, when acute myeloid leukemia cells (Nervi et al., 2009) and multiple myeloma cells (Azab et al., 2009) are treated with a C-X-C chemokine receptor type 4 (CXCR4) inhibitor, known to prevent the lodging of cancer cells into select microenvironments prior to chemotherapy, the chemosensitivity of these cells was dramatically enhanced. In part, release from the protection of the microenvironmental niche could sensitize CSCs to chemotherapeutics. Additionally, it is possible that disruption of CXCL12/CXCR4 signaling may activate CSC cycling which in turn could sensitize CSCs to agents targeting proliferating cells. For targeting solitary and potentially dormant DTCs in patients with no clinically apparent distant disease, this strategy has distinct advantages as an adjuvant therapy to prevent metastasis.
8. Future prospects
The conceptual framework of CSCs is likely to be integral to furthering our understanding of the origin of tumors and identifying targets for curative therapies. However, not only is targeting CSCs not likely to be easy, but targeting only CSCs may not be enough to prevent metastasis or relapse due to metastasis. Continued development of combination therapies with multiple targets (e.g. targeting CSCs, combination of chemotherapy, differentiation therapy, targeting microenvironment) will remain essential in order to improve outcomes of individuals with solid tumors. Nevertheless, site-specific dissemination and activation of CSCs provides a mechanistic explanation for the evolution of many metastases, as well as for metastatic inefficiency (Paget, 1889). In fact, given the growth limitations known to restrict the uncontrolled growth of tumor cells, most occult metastases may require months and even years before they can be identified and treated (Kendal, 2010). As most neoplasms are identified after they have reached a critical mass, the ability to block the reactivation of dormant CSCs at distant sites of metastases is a critical area of research. Therapies based on this concept are currently not at hand and essential aspects of the current dormancy model must be validated. However these data support the need for further research directed at preventing metastases from developing by targeting CSCs within primary and disseminated sites and to slow or limit CSC growth when necessary.
Acknowledgements
We thank Drs McCauley, Keller (University of Michigan) for helpful discussions. Figures were produced using Servier Medical Art (www.servier.com). This work is directly supported by the National Cancer Institute (CA163124, CA093900, Y.S., K.J.P and R.S.T.), the Fund for Cancer Research (R.T.), the Department of Defense (R.S.T.), and the Prostate Cancer Foundation (Y.S., K.J.P and R.S.T.) the NIH SPORE in prostate cancer grant P50 CA69568 (K.J.P, R.T.).
Abbreviations
Abbreviation Definition
- ABC
Adenosine triphosphate-binding cassette
- ALDH
Aldehyde dehydrogenase
- APL
Acute promyelocytic leukemia
- BMP
Bone morphogenic protein
- CEA
Carcinoembryonic antigen
- CSC
Cancer Stem cell
- CTCs
Circulating tumor cells
- CXCL12
Stromal derived factor-1 (SDF-1 or CXCL12)
- DTC
Disseminated tumor cells
- EMT
Epithelial to mesenchymal transition
- GAS6
Growth arrest specific-6
- HSC
Hematopoietic stem cell
- miRNA
microRNAs
- (NOD)/SCID IL2Rγnull mice
Non-obese diabetic SCID mice lacking the IL-2 receptor-mice-gamma
- NSC
Normal stem cells
- PCa
Prostate cancer
- PKC
Protein kinase C
- PTEN
Phosphatase and tensin homolog
- SCID
Severe combined immune deficient mice
- TNC
Tenacin C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Conflict of Interest
None
Reference List
- Adikrisna R, Tanaka S, Muramatsu S, Aihara A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Yamaoka S, Arii S. Identification of pancreatic cancer stem cells and selective toxicity of chemotherapeutic agents. Gastroenterology. 2012;143:234–245. doi: 10.1053/j.gastro.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc.Natl.Acad.Sci.U.S.A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert.Opin.Drug Metab Toxicol. 2009;5:1529–1542. doi: 10.1517/17425250903228834. [DOI] [PubMed] [Google Scholar]
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol.Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atala A. Re: Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. J.Urol. 2012;187:1506–1507. doi: 10.1016/j.juro.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype 22. Clin.Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- Bautch VL. Stem cells and the vasculature. Nat.Med. 2011;17:1437–1443. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18:370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors 1. Nat.Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr. Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies 1. Med.Res Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de RM, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, Longaker MT, Weissman IL. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AK, Hills RK, Green C, Jenkinson S, Koo K, Patel Y, Guy C, Gilkes A, Milligan DW, Goldstone AH, Prentice AG, Wheatley K, Linch DC, Gale RE. The impact on outcome of the addition of all-trans retinoic acid to intensive chemotherapy in younger patients with nonacute promyelocytic acute myeloid leukemia: overall results and results in genotypic subgroups defined by mutations in NPM1, FLT3, and CEBPA. Blood. 2010;115:948–956. doi: 10.1182/blood-2009-08-236588. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells 1. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cheung JY, Miller BA. Molecular mechanisms of erythropoietin signaling. Nephron. 2001;87:215–222. doi: 10.1159/000045918. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Research. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LW, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J.Clin.Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Research. 2005a;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Research. 2005b;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N.Engl.J.Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, Scher HI. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin.Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- de Sousa E Melo, Colak S, Buikhuisen J, Koster J, Cameron K, de Jong JH, Tuynman JB, Prasetyanti PR, Fessler E, van den Bergh SP, Rodermond H, Dekker E, van der Loos CM, Pals ST, van de Vijver MJ, Versteeg R, Richel DJ, Vermeulen L, Medema JP. Methylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell. 2011;9:476–485. doi: 10.1016/j.stem.2011.10.008. [DOI] [PubMed] [Google Scholar]
- de Sousa EM, Vermeulen L, Richel D, Medema JP. Targeting Wnt signaling in colon cancer stem cells. Clin.Cancer Res. 2011;17:647–653. doi: 10.1158/1078-0432.CCR-10-1204. [DOI] [PubMed] [Google Scholar]
- Dieter SM, Ball CR, Hoffmann CM, Nowrouzi A, Herbst F, Zavidij O, Abel U, Arens A, Weichert W, Brand K, Koch M, Weitz J, Schmidt M, von Kalle C, Glimm H. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases 2. Cell Stem Cell. 2011;9:357–365. doi: 10.1016/j.stem.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Ding XW, Wu JH, Jiang CP. ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sci. 2010;86:631–637. doi: 10.1016/j.lfs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolyers that overexpress growth arrest-specific gene-6 (GAS6). Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12260–12265. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nature Immunology. 2005;6(3):314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population 4. Cell Death.Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Research. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Research. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1 5. Nat.Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening 1. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, Neeley C, Wang J, Mehra R, Keller ET, McCauley LK, Loberg RD, Pienta KJ, Taichman RS. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–380. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylock DN, Nilsson SK. Stem cell regulation by the hematopoietic stem cell niche. Cell Cycle. 2005;4:1353–1355. doi: 10.4161/cc.4.10.2056. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, Fitch-Bruhns M, Lazetic S, Park IK, Sato A, Satyal S, Wang X, Clarke MF, Lewicki J, Gurney A. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency1. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 2009;27:844–850. doi: 10.1080/07357900902744502. [DOI] [PubMed] [Google Scholar]
- Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, Ward T, Blackhall FH, Dive C. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J.Clin.Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der HM, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J, Tabar V. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol.Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat.Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, Gearing DP, Vairo G, Lopez AF, Dick JE, Lock RB. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Joseph J, Shiozawa Y, Jung Y, Kim JK, Pedersen E, Mishra A, Zalucha JL, Wang J, Keller ET, Pienta KJ, Taichman RS. Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Mol.Cancer Res. 2012;10:282–292. doi: 10.1158/1541-7786.MCR-11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Berry JE, Havens AM, Joseph J, Kim JK, Patel L, Carmeliet P, Daignault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia. 2012a;14:429–439. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Havens A, Joseph J, Kim JK, Patel LR, Carmeliet P, Diagnault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS. Prevalence of Prostate Cancer Metastases Following Intravenous Inoculation Provides Clues into the Molecular Basis of Dormancy in the Bone Marrow Microenvironment. Neoplasia. 2012b;14(5):429–39. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalikin LM, Schneider A, Thakur MA, Fridman Y, Griffin LB, Dunn RL, Rosol TJ, Shah RB, Rehemtulla A, McCauley LK, Pienta KJ. In vivo visualization of metastatic prostate cancer and quantitation of disease progression in immunocompromised mice.[see comment]. Cancer Biology & Therapy. 2003 Dec.2(6):656–60. [PubMed] [Google Scholar]
- Kang YB, Siegel PM, Shu WP, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol.Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Research. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005a;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche.[see comment]. Nature. 2005b;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells 1. Breast Cancer Res. 2012;14:R15. doi: 10.1186/bcr3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor Growth Need Not Be Driven by Rare Cancer Stem Cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kendal WS. Extinction kinetics for metastatic cancer stem cells. Int.J.Radiat.Biol. 2010;86:918–926. doi: 10.3109/09553002.2010.492491. [DOI] [PubMed] [Google Scholar]
- King CE, Cuatrecasas M, Castells A, Sepulveda AR, Lee JS, Rustgi AK. LIN28B promotes colon cancer progression and metastasis. Cancer Research. 2011;71:4260–4268. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. [Review] [69 refs]. Physiology. 1920:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. [Review] [166 refs]. Stem Cells. 2005;23(7):879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, Daley GQ, Doxsey S. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity 3. Nat.Cell Biol. 2011;13:1214–1223. doi: 10.1038/ncb2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Lee CG, Das B, Lin TL, Grimes C, Zhang X, Lavezzi T, Huang L, Cole J, Yau L, Li L. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. British Journal of Haematology. 2012;158:79–90. doi: 10.1111/j.1365-2141.2012.09123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice 18. Hum.Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Li Y, Laterra J. Cancer stem cells: distinct entities or dynamically regulated phenotypes?. Cancer Research. 2012;72:576–580. doi: 10.1158/0008-5472.CAN-11-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, Xia S, Trageser D, Guerrero-Cazares H, Eberhart CG, Quinones-Hinojosa A, Scheffler B, Laterra J. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc.Natl.Acad.Sci.U.S.A. 2011;108:9951–9956. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends in Biochemical Sciences. 2006;31(10):589–95. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat.Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, Clarke MF. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc.Natl.Acad.Sci.U.S.A. 2010;107:18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Research. 2009;69:8536–8539. doi: 10.1158/0008-5472.CAN-09-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, Murphy GF, Waaga-Gasser AM, Gasser M, Stephen HF, Frank NY, Frank MH. Isolation of tumorigenic circulating melanoma cells. Biochem.Biophys.Res Commun. 2010;402:711–717. doi: 10.1016/j.bbrc.2010.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Marsden CG, Wright MJ, Pochampally R, Rowan BG. Breast tumor-initiating cells isolated from patient core biopsies for study of hormone action. Methods Mol.Biol. 2009;590:363–375. doi: 10.1007/978-1-60327-378-7_23. [DOI] [PubMed] [Google Scholar]
- Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, O'Brien T, Kerin MJ. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res.Treat. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann.Oncol. 2006;17:1620–1624. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat.Med. 2005;11:1026–1028. doi: 10.1038/nm1005-1026. [DOI] [PubMed] [Google Scholar]
- Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Research. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Nagai K, Hoshino H, Mimori K, Tanaka F, Nagano H, Sekimoto M, Doki Y, Mori M. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc.Natl.Acad.Sci.U.S.A. 2010;107:40–45. doi: 10.1073/pnas.0912407107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J, Fodde R. Cancer stemness and metastasis: therapeutic consequences and perspectives. Eur.J.Cancer. 2010;46:1198–1203. doi: 10.1016/j.ejca.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Research. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, DiPersio JF. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what's in the name?. Biochem.Biophys.Res Commun. 2007;355:855–859. doi: 10.1016/j.bbrc.2007.01.159. [DOI] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat.Rev.Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Osawa M, Yonetani S, Torikai-Nishikawa S, Freter R. Niche required for inducing quiescent stem cells. Cold Spring Harb.Symp.Quant.Biol. 2008;73:67–71. doi: 10.1101/sqb.2008.73.024. [DOI] [PubMed] [Google Scholar]
- Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115:3704–3707. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat.Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- Pallini R, Ricci-Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M, Stassi G, Martini M, Maira G, Larocca LM, De Maria R. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin.Cancer Res. 2008;14:8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- Pantel K, Alix-Panabieres C. The clinical significance of circulating tumor cells. Nat.Clin.Pract.Oncol. 2007;4:62–63. doi: 10.1038/ncponc0737. [DOI] [PubMed] [Google Scholar]
- Patel LR, Shiozawa Y, Taichman RS, Pienta KJ. CD133+/CD44+ Cancer Stem Cells Represent a Disproportionately Large Fraction of Early Bone Disseminated Prostate Cancer Tumor Cells. A. 2010 [Google Scholar]
- Patt HM, Maloney MA, Lamela RA. Hematopoietic stem cell proliferative behavior as revealed by bromodeoxyuridine labeling. Experimental Hematology. 1980;8:1075–1079. [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat.Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME. Regulating cancer stem cells the miR way. Cell Stem Cell. 2010;6:4–6. doi: 10.1016/j.stem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma 46. Proc.Natl.Acad.Sci.U.S.A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Rhim JS, Li H, Furusato B. Novel human prostate epithelial cell culture models for the study of carcinogenesis and of normal stem cells and cancer stem cells. Adv.Exp.Med.Biol. 2011;720:71–80. doi: 10.1007/978-1-4614-0254-1_6. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues.Organs. 1999;165:190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, Tsukuda K, Yamane M, Oto T, Kiura K, Miyoshi S. Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer. 2012;77:162–167. doi: 10.1016/j.lungcan.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008a;105:370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008b doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J.Clin.Invest. 2011a;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pienta KJ, Taichman RS. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin.Cancer Res. 2011c doi: 10.1158/1078-0432.CCR-10-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, Smith C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc.Natl.Acad.Sci.U.S.A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sun YX, Pedersen EA, Shiozawa Y, Havens AM, Jung Y, Wang J, Pienta KJ, Taichman RS. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin.Exp.Metastasis. 2008;25(7):765–76. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal Localization and Neutralization of the SDF-1(CXCL12)/CXCR4 Axis Blocks Prostate Cancer Metastasis and Growth in Osseous Sites In Vivo. Journal of Bone & Mineral Research. 2005:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, Marquez VE, Clement V, Stamenkovic I. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Research. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman N, McCauley LK. Use of the Stromal Cell-derived Factor-1/CXCR4 Pathway in Prostate Cancer Metastasis to Bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem cell niche. Blood. 2005;1052631-9(7):2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos PA, Polioudaki H, Agelaki S, Kallergi G, Saridaki Z, Mavroudis D, Georgoulias V. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288:99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- Trosko JE. Review paper: cancer stem cells and cancer nonstem cells: from adult stem cells or from reprogramming of differentiated somatic cells. Vet.Pathol. 2009;46:176–193. doi: 10.1354/vp.46-2-176. [DOI] [PubMed] [Google Scholar]
- van der PG. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48:37–43. doi: 10.1016/j.bone.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, de Sousa MF, Sprick MR, Kemper K, Perez AM, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc.Natl.Acad.Sci.U.S.A. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chen ZG, Du CZ, Wang HW, Yan L, Gu J. Cancer stem cell marker CD133+ tumour cells and clinical outcome in rectal cancer. Histopathology. 2009;55:284–293. doi: 10.1111/j.1365-2559.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes and Development. 2008;22:559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo T, Okudela K, Mitsui H, Yazawa T, Ogawa N, Tajiri M, Yamamoto T, Rino Y, Kitamura H, Masuda M. Prognostic value of CD133 expression in stage I lung adenocarcinomas. Int.J.Clin.Exp.Pathol. 2010;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R, Wunder JS, Alman BA. Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Research. 2007;67:8216–8222. doi: 10.1158/0008-5472.CAN-07-0999. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Yu C, Yao Z, Dai J, Zhang H, Escara-Wilke J, Zhang X, Keller ET. ALDH activity indicates increased tumorigenic cells, but not cancer stem cells, in prostate cancer cell lines. In Vivo. 2011a;25:69–76. [PubMed] [Google Scholar]
- Yu F, Li J, Chen H, Fu J, Ray S, Huang S, Zheng H, Ai W. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011b;30:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp.Gerontol. 2008;43:981–985. doi: 10.1016/j.exger.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod.Pathol. 2012;25:456–464. doi: 10.1038/modpathol.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Niu C, Ye L, Huang HY, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li LH. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, III, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc.Natl.Acad.Sci.U.S.A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004 2004 Jun;26(6):595–9. doi: 10.1002/bies.20052. BioEssays. 26, 595.-599. [DOI] [PubMed] [Google Scholar]