Abstract
The herbicide 2,6-dichlorobenzonitrile (DCBN) is a potent nasal toxicant in rodents; however it is not known whether DCBN causes similar nasal toxicity in humans. The tissue-selective toxicity of DCBN in mouse nasal mucosa is largely dependent on target tissue bioactivation by CYP2A5. The human orthologs of CYP2A5, CYP2A6 and CYP2A13, are both expressed in nasal mucosa, and are capable of activating DCBN. In this study, we directly determined the ability of human nasal mucosa to bioactivate DCBN. We also tested the suitability of a glutathione conjugate of DCBN (GS-DCBN) or its derivatives as biomarkers of DCBN exposure and nasal toxicity in mouse models. We found that human fetal nasal-mucosa microsomes catalyze the formation of GS-DCBN, with a Km value comparable to that of adult mouse nasal-mucosa microsomes. The activity of the human nasal-mucosa microsomes was inhibited by 8-methoxypsoralen, a known CYP2A inhibitor. GS-DCBN and its metabolites were detected in the nasal mucosa and nasal-wash fluid obtained from DCBN-treated mice, in amounts that increased with escalations in DCBN dose, and they were all still detectable at 24 h after a DCBN treatment (at 10 mg/kg). Further studies in Cyp2a5-null mice indicated that GS-DCBN and its metabolites in nasal-wash fluid were generated in the nasal mucosa, rather than in other organs. Thus, our data indicate for the first time that the human nasal mucosa is capable of bioactivating DCBN, and that GS-DCBN and its metabolites in nasal-wash fluid may collectively serve as indicators of DCBN exposure and potential nasal toxicity in humans.
Keywords: Nasal toxicity, dichlobenil, olfactory mucosa, CYP2A5, glutathione conjugates, biomarkers
Introduction
The herbicide 2,6-dichlorobenzonitrile (DCBN) is one of the most potent nasal toxicants in rodents.1 DCBN induces permanent loss of olfactory neuroepithelium in the dorsal medial region of the nasal cavity, following a single exposure at relatively low doses, in spite of the remarkable regeneration capacity of the neuronal stem cells residing in the olfactory mucosa.2,3 However, it remains unknown whether DCBN induces nasal toxicity in humans, who are potentially exposed to DCBN in an occupational setting, or through environmental contamination. Currently, there are no appropriate biomarkers to monitor DCBN exposure and its nasal toxicity in humans.
We have recently demonstrated that while hepatic P450-catalyzed metabolism is essential for the systemic clearance of DCBN, nasal CYP2A5-catalyzed metabolic activation is responsible for DCBN-induced olfactory toxicity in mice.4 It has been reported that CYP2A6 and CYP2A13, which are human orthologs of mouse CYP2A5, are both expressed in human nasal tissues, and recombinant CYP2A6 and CYP2A13 were both active in a reconstituted system in metabolizing DCBN to form protein adducts.5,6 However, the ability of P450 enzymes contained in human nasal tissues to bioactivate DCBN has not been characterized.
The tissue specific nature of the DCBN toxicity and the requirement for target tissue bioactivation in the nasal mucosa suggest that urinary metabolites of DCBN are unlikely suitable as biomarkers for assessing potential DCBN nasal toxicity, although they may reflect the levels of systemic exposure and thus possible tissue burden. Nonetheless, if DCBN exposure is through inhalation, the levels of DCBN in the nasal mucosa will unlikely be reflected by circulating DCBN levels.
The olfactory toxicity of DCBN is mediated by reactive intermediates that are formed during its metabolic activation catalyzed by P450s.7 It has been proposed that, once reaching nasal mucosa, DCBN is activated by P450s (mainly CYP2A5 in mice) to form epoxide intermediates, which can conjugate glutathione or bind nucleophilic parts of proteins and form protein adducts, resulting in tissue damages.8 This mechanism is supported by the observation of significant depletion of non-protein thiol (mainly GSH) in mouse nasal mucosa at two hours after an intraperitoneal injection of DCBN.4 The extent of this depletion was dose-dependent and related to the degree of final pathological changes of nasal mucosa in mice.4,9 Therefore, we hypothesized that the GSH conjugate of DCBN (GS-DCBN) or its derivatives can serve as biomarkers of DCBN exposure and potential toxicity. In fact, detection of GSH-trapped drug metabolites is an approach widely used for the rapid screening of potentially toxic metabolites in the drug discovery process in pharmaceutical industry.10 On the other hand, detection of urinary mercapturic acids, metabolites of GSH conjugates, is generally used for the assessment of exposure to electrophilic chemicals in occupational monitoring.11
In the current study, we first established a sensitive LC-MS/MS method to detect and quantify GS-DCBN and its metabolites in biological matrices. Using this method, we determined the kinetic parameters of GS-DCBN formation catalyzed by human fetal nasal microsomes and compared them to that catalyzed by mouse nasal mucosa microsomes. We then analyzed in vivo samples collected from mice treated with DCBN at different doses through different routes. Our results showed that GS-DCBN and its metabolites are detectable not only in nasal tissues, but also in nasal-wash fluid, after treatment of mice with DCBN, even at sub-toxic doses, in a dose-dependent and toxicity-related manner. These results suggest that the levels of GS-DCBN and its metabolites in nasal-wash fluid could be biomarkers of DCBN exposure and potential nasal toxicity in humans.
Materials and Methods
Chemicals and Enzymes
DCBN (97% pure), 2,6-[ring-14C]DCBN (20.1 Ci/mol, >99% pure), L-glutathione (GSH), γ-L-glutamyl-L-cysteine (Gly-Cys, >85% pure), L-cysteine (Cys), N-acetyl-L-cysteine (N-acetyl-Cys) and 8-methoxyporsalen were from Sigma-Aldrich (St. Louis, MO). Stable isotope-labeled GSH ([Glycine-13C2 (>98%), 15N (96–99%)]GSH, or [13C2-15N]GSH) was from Cambridge Isotope Laboratories (Andover, MA). High-performance liquid chromatography (HPLC)-grade acetonitrile, methanol, and water were from Fisher Scientific (Pittsburgh, PA). Heterologously expressed human cytochrome P450 reductase was from Invitrogen (Carlsbad, CA). Heterologously expressed mouse CYP2A5 was prepared as described.12
In Vitro Metabolism of DCBN
Mouse microsomal samples were prepared from pooled nasal mucosa of 10–12 mice, as described previously.13 Human fetal nasal mucosa was provided by the University of Washington Birth Defect Research Laboratory (Seattle, WA). Fetal nasal mucosa microsomes were prepared as described previously,14 from pooled nasal tissues of 8 fetuses (5 male and 3 female, with gestational ages of 104–120 days). Microsomes were stored at −80 °C prior to use. In vitro reactions were carried out as previously reported,8,12 with some modifications. The contents of reaction mixtures and the incubation conditions are indicated in the legends to figures and tables. Reactions were initiated with the addition of NADPH, and were terminated by the further addition of 40 μl of Solution P, containing methanol/perchloric acid/water (90:3.5:6.5, v/v/v). After centrifugation at 1,200g for 10 min, the supernatant was analyzed by either liquid chromatography-mass spectrometry (LC-MS), or radiometric HPLC, for determination of metabolite formation. Rates were linear with incubation time under the conditions used.
Instrumental Analysis of DCBN Conjugates
The MS analyses were performed using an Applied Biosystems/MDS Sciex API 4000 Q-Trap tandem mass spectrometer equipped with a turbo spray ion source and interfaced with an Agilent 1200 series liquid chromatograph. Radiometric detection was accomplished as described previously8 by using a Radiomatic model A-500 detector (Packard) interfaced with an Agilent 1100 series liquid chromatograph. Nova-Pak C18 columns (3.9 × 75 mm, 4-μm, Waters, Milford, MA) were used for analyses. HPLC mobile phase consisted of A [formic acid (0.1%) in water/acetonitrile (90:10, v/v)] and B [methanol (100%)], with a flow rate of 0.3 ml/min. The HPLC gradient started at 100% A for 3 min, ramped linearly to 100% B over 20 min, held at 100% B for 3 min, and returned to the initial condition over 4 min. The column was re-equilibrated for 10 min before the next injection. The mass spectrometer was operated in the positive ionization mode. Data were analyzed with the Analyst software (version 1.4.2, Applied Biosystems/MDS Sciex). Data from the radiometric detector were processed using the Flo-One software (Packard).
For neutral loss-enhanced product ion (NL-EPI) analysis, the NL scan (for m/z 129) was performed using a scan range of m/z 150–600, a 0.2-Da step size, a 5-ms pause between cycles, and a 2-s scan time. The ion source parameters were optimized and set as the following arbitrary values: curtain gas, 35; collision gas, medium; ionspray voltage, 4500; temperature, 500; ion source gas 1, 55; and ion source gas 2, 60. Nitrogen was used as the nebulizer and auxiliary gas. Information-dependent acquisition was used to trigger acquisition of EPI spectra for ions exceeding 5000 cps, with exclusion of former target ions after three occurrences for 10 s.15 The EPI scan was performed with a scan range (for daughter ions) of m/z 50–800; the other conditions were set as: scan mode, profile; step size, 0.08 Da; scan rate, 1000 Da/s; and pause between cycles, 5 ms.15 Multiple reaction monitoring (MRM) was used for quantitative analysis. The monitored transitions, selected on the basis of EPI spectra, were: m/z 459→200, for GS-DCBN; m/z 330→200, for Gly-Cys-DCBN; m/z 273→156, for Cys-DCBN; m/z 315→200, for N-acetyl-Cys-DCBN; and m/z 462→200, for [13C2-15N]GS-DCBN (internal standard). Nitrogen was used as the curtain gas (setting at 35), gas 1 (setting at 35), gas 2 (setting at 60), and the collision gas (setting medium). The ion spray voltage was set at 4800, and the gas temperature was set at 450°C. The declustering, entrance, and collision exit potentials were 60, 10, and 12 V, respectively. The collision energy was 40 eV for GS-DCBN, Gly-Cys-DCBN, and the internal standard and 42 eV for Cys-DCBN and N-acetyl-Cys-DCBN.
Animal treatments
Male and female wild-type C57BL/6 (WT B6) and Cyp2a5-null mice (on B6 genetic background), 2–3 months of age, were obtained from breeding stocks maintained at the Wadsworth Center. Animal use protocols were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. Mice were treated with either a single intraperitoneal dose of DCBN at 5 or 10 mg/kg, or multiple subcutaneous doses at 1 mg/kg each (5 μl/g body weight); DCBN was dissolved in a mixture of dimethyl sulfoxide (DMSO; from Calbiochem) and corn oil (1:4; v/v). Control mice were injected with vehicle only. Animals were dosed at 10:00–11:00 AM, local time. Blood, nasal wash, and nasal mucosa samples were collected from individual mouse at a series of time points after dosing (30 min, and 2, 6, and 24 h). The animals were sacrificed by CO2 overdose. Nasal wash samples were collected by flushing mouse nasal cavity at room temperature with 1.2 ml of phosphate-buffered saline (PBS), essentially as reported.16 Briefly, after removal of the mandible, the nasal cavity was gently flushed with PBS from a posterior opening of the nose, and nasal wash samples were collected from the anterior openings of the nose. The nasal wash samples were centrifuged at 13,000g for 10 min to remove any contaminating blood cells. nasal mucosa tissue samples were dissected following the nasal wash procedure. Blood samples, collected through cardiac puncture, were kept on ice for 1 h prior to centrifugation at 13,000g for 10 min at 4 °C. Nasal wash, nasal mucosa, and serum samples were stored at −80 °C until use.
Preparation of GS-DCBN and other metabolite standards
Heterologously expressed CYP2A5 was utilized to generate the standards of GS-DCBN and its further metabolites. Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 1 μM DCBN or [ring-14C]DCBN, added in 1 μl methanol, 0.1 mM CYP2A5,12 0.3 mM P450 reductase, 5 mM GSH (or Gly-Cys, or Cys, or N-acetyl-Cys), and 1 mM NADPH, in a final volume of 0.1 ml. For the generation of the internal standard, [13C2-15N]GSH was used to substitute GSH. The reactions were carried out at 37°C for 30 min, and terminated by the addition of 40 μl of solution P. In control incubations, NADPH was omitted. The standards generated were collected from the supernatant after centrifugation at 1,200g for 10 min. Metabolites generated in incubations with DCBN were detected by LC-MS, whereas metabolites generated in incubations with [ring-14C]DCBN were detected using radiometric HPLC, using identical HPLC conditions, as described above. The quantities of the 14C-labeled standards generated, calculated based on the specific radioactivity of the labeled substrate, were taken as the amounts of the non-radiolabeled standards generated in parallel incubations with non-radiolabeled DCBN, for quantification of metabolites detected by LC-MS.
Sample Preparation for Determination of GS-DCBN and Other in vivo Metabolites
nasal mucosa samples were thawed and weighed at room temperature, and then homogenized in 500 μl of 100 mM Tris-acetate buffer, pH 7.4, containing 1.0 mM EDTA and 150 mM potassium chloride, with use of a Bullet Blender™ (Next Advance, Averill Park, NY), as described previously 4. For metabolite determination, 400 μl of nasal mucosa homogenate, 1 ml of nasal wash samples, or 50 μl of serum from DCBN-treated or untreated (blank; for preparation of calibrators) mice, were used. All samples were fortified with 0.34 pmol of [13C2-15N]GS-DCBN. The nasal mucosa samples, serum samples, and calibrators were extracted with 2 vol of methanol. After centrifugation at 13,000g for 10 min, the supernatant was diluted with 2.5 ml of water and then purified by solid-phase extraction (SPE) on Isolute C18 cartridges (200 mg/3 ml, Biotage, Charlottesville, VA). The nasal wash samples were directly purified through SPE. The C18 cartridges were first activated with 3 ml of methanol and then equilibrated with 3 ml of water. After sample loading, the analytes were eluted in 2 ml of methanol, dried under nitrogen, resuspended in 100 μl of a 30:70 (v/v) mixture of solution P and potassium phosphate buffer (50 mM, pH 7.4), and then analyzed by LC-MS/MS. The recovery of SPE cleaning has been tested to be over 80%.
Calibration Curves and Method Validation
For quantitative analysis of GS-DCBN and its metabolites in biological samples, calibration curves (over the ranges of 8.45-135 pmol/ml for GS-DCBN, 1.25–20 pmol/ml for Gly-Cys-DCBN, 2.4–38.4 pmol/ml for Cys-DCBN, and 8–129 pmol/ml for N-acetyl-Cys-DCBN) were prepared, with the use of 50 μl blank serum as the matrix. The calibration curves were plotted with the use of concentration ratios (analytes to internal standard) as the x axis and peak area ratios (analytes to internal standard) as the y axis.
The method for quantification of GS-DCBN and its metabolites in tissue and serum samples was validated by determination of intraday and interday precision and accuracy. For each batch, 50 μl blank serum was fortified with a fixed amount of the internal standard (0.34 pmol of [13C2-15N]GS-DCBN) and spiked with appropriate amounts of metabolite standards to achieve the following concentrations: GS-DCBN, 13.5 and 54 pmol/ml; Gly-Cys-DCBN, 2.5 and 10 pmol/ml; Cys-DCBN, 5 and 20 pmol/ml; and N-acetyl-Cys-DCBN, 15 and 60 pmol/ml. The samples were extracted, cleaned through SPE, and analyzed by LC-MS/MS (using MRM). Three validation batches were prepared; each batch, including four replicates of samples spiked with each concentration, was analyzed on a different day.
Other Methods
Pharmacokinetic parameters, including area under concentration-time curve (AUC), maximum plasma concentration (Cmax), and elimination half-time (t1/2) were calculated using PKSolver, an add-in program in Microsoft Excel.17 Histopathological examination of the nasal mucosa was carried out as described previously.4 Statistical analysis for goodness of fit (for analysis of enzyme kinetics) was performed using Graphpad Prism 5 (Graphpad, San Diego, CA). Student’s t-test was selected for comparisons between two groups, whereas one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test was used to compare among multiple groups, with use of SigmaStat 2.0 (Systat, Chicago, IL).
Results
Detection of GS-DCBN by NL-EPI scanning
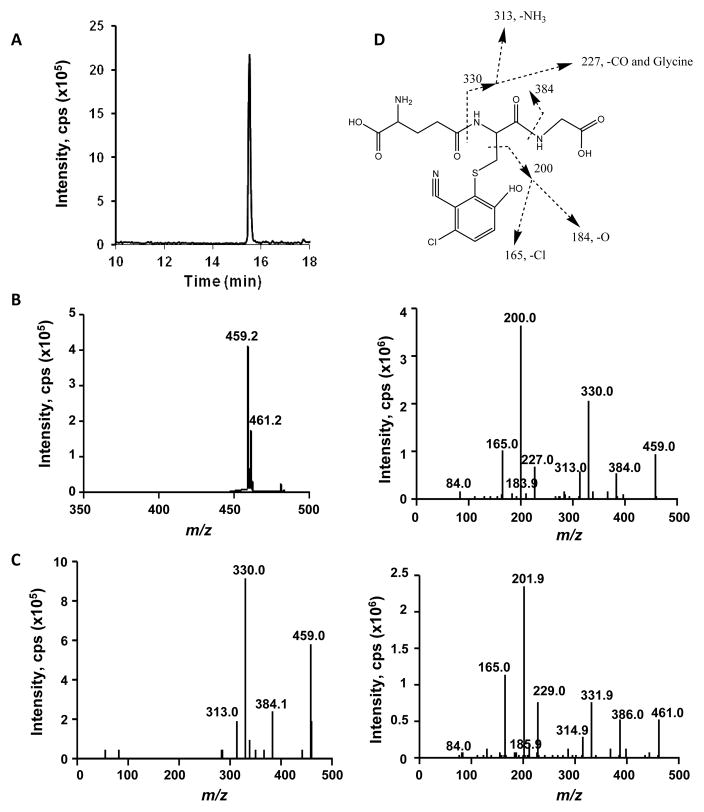
The LC-MS/MS method for GS-DCBN detection was first developed using incubation mixtures of DCBN with mouse nasal mucosa microsomes and saturating amounts of GSH (Figure 1). A single GSH conjugate (GS-DCBN) was observed in the total ion chromatogram of the NL scan for 129 Da (Figure 1A); no peak was detected in incubation mixtures without NADPH (data not shown). The m/z values (459.2 and 461.2; Figure 1B) for the peak shown in Figure 1A reconciled well with those proposed previously for GS-DCBN,8 whereas the relative abundance of the two ions (459.2:461.2 = ~3) was consistent with the existence of one chlorine in the molecule. The NL-directed EPI scan showed characteristic fragments of GSH conjugates (Figure 1C). More comprehensive fragmentation information was obtained by EPI scans for m/z 459 (Figure 1D, middle panel) and m/z 461 (Figure 1D, bottom panel) parent ions, which formed the bases for the proposed fragmentation pathway for GS-DCBN (Figure 1D, top panel).
Fig 1. LC-MS/MS detection and identification of GS-DCBN.
GS-DCBN was produced in incubations of DCBN with nasal mucosa microsomes from WT mice. Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 30 μM DCBN, added in 1 μl methanol, 0.5 mg/ml nasal mucosa microsomal protein from 2-month-old male mice, 5 mM GSH, and 1 mM NADPH, in a final volume of 0.1 ml. The reaction was carried out at 37 °C for 10 min. In control incubations, NADPH was omitted. (A) Chromatogram of GSH conjugates detected by LC-MS/MS using the neutral loss scanning for m/z 129, in the positive ion mode; (B) MS spectrum of the peak detected in (A); (C) MS/MS spectrum obtained using NL-EPI analysis of the peak detected in (A); (D) LC-ESI-MS/MS product ion spectra of GS-DCBN standard at m/z 459 and m/z 461, in the positive ion mode. Proposed fragmentation of the [M+H]+ ion of GS-DCBN is also shown.
Detection of further metabolites of GS-DCBN in mouse nasal mucosa
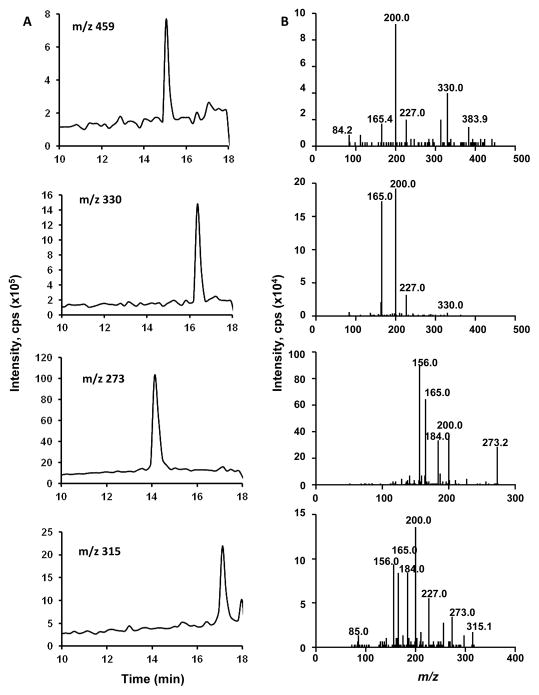
GSH conjugates can be further metabolized sequentially by γ-glutamyltransferase (a dipeptidase) and cysteine S-conjugate N-acetyltransferase to a series of sulfur-containing metabolites.11 For GS-DCBN, the predicted metabolites (Gly-Cys-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN) and corresponding m/z values are shown in Figure 2. These GS-DCBN metabolites, which should be detectable using the same NL-EPI scanning method that was used for detection of GS-DCBN, were not detected in incubations of DCBN with mouse nasal mucosa microsomes (Figure 1A), consistent with the cytosolic localization of the transferases. However, in nasal mucosa tissue homogenates from DCBN-injected mice, GS-DCBN and all three of its metabolites were detected by EPI scanning for their respective m/z values (Figure 3).
Fig 2.
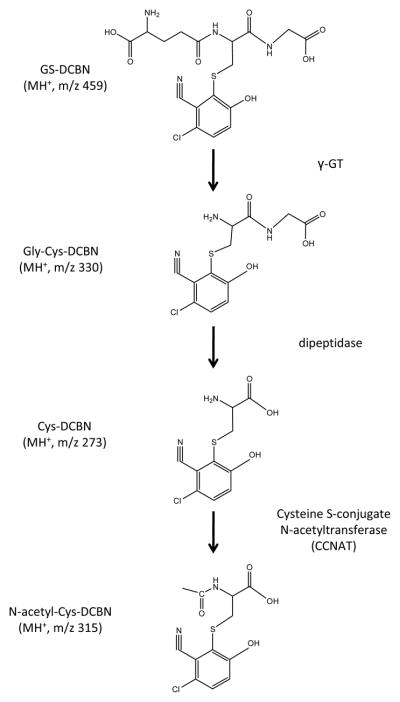
Schematic representation of the catabolism of GS-DCBN and structures of the detected metabolites
Fig 3. LC-MS/MS analysis of DCBN metabolites detected in the nasal mucosa of DCBN-treated mice.
Two- to three-month old WT mice were treated with a single i.p. injection of DCBN (at 25 mg/kg). Nasal mucosa was dissected at 2 h after the injection. (A) Extracted ion chromatograms of [M+H]+ ion at m/z 459, m/z 330, m/z 257, and m/z 315, respectively; (B) LC-ESI-MS/MS product ion spectra of the respective [M+H]+ ions shown in panel (A).
Quantification of GS-DCBN and its metabolites
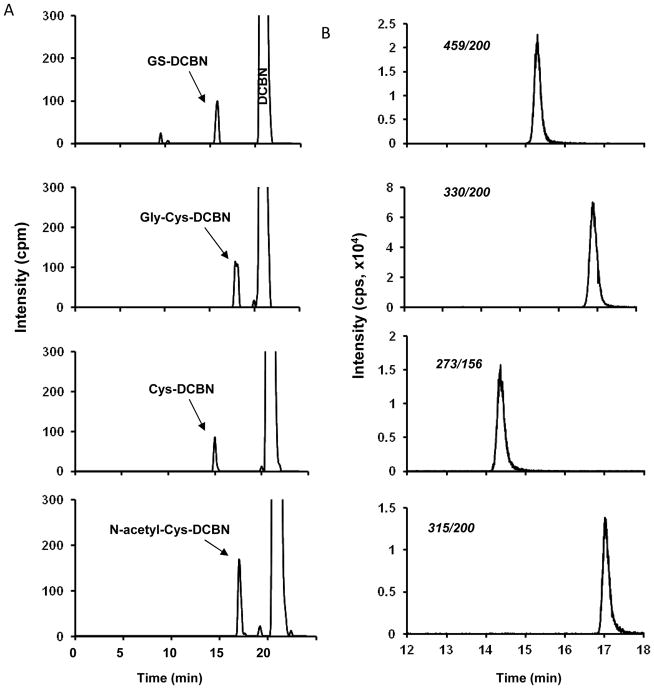
Standards for GS-DCBN and its metabolites are not commercially available. Our previous study has demonstrated that CYP2A5 is the major P450 responsible for DCBN bioactivation in mouse nasal mucosa.4 Therefore, we utilized heterologously expressed CYP2A5 to generate standards for GS-DCBN and its metabolites, by adding saturating amounts of GSH, Gly-Cys, Cys, or N-acetyl-Cys to reaction mixtures of CYP2A5 with either unlabeled DCBN or [ring-14C]DCBN, as described in the method. The radiolabeled and non-radiolabeled DCBN metabolites, produced in parallel reactions under identical conditions, were respectively analyzed by radiometric HPLC (Figure 4A) and LC-MS/MS (Figure 4B), using identical HPLC conditions. As shown in Figure 4, for each set of reactions, metabolite peaks of identical retention times were detected both on radiometric detector and by the MRM transition (selected for each analyte on the basis of their corresponding product ion spectrum; Figure 3B). Thus, the MS-based detection provided structural confirmation for the metabolites detected, while the radiometric analysis showed the amounts of the metabolites formed, based on the specific radioactivity of the parent compound, [ring-14C]DCBN. The retention times for each metabolite were unique, and thus would allow baseline separation of the peaks. The same approach was successfully used to generate (by replacing GSH with [13C2-15N]GSH in the reaction mixture) and quantitate the internal standard, [13C2-15N]GS-DCBN.
Fig 4. Enzymatic synthesis and quantification of metabolite standards for GS-DCBN and its derivatives.
Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 1 μM DCBN (including 2,6-[ring-14C]DCBN, 20.1 Ci/mol, for samples to be analyzed by radiometric LC), added in 1 μl of methanol, a reconstituted P450 system consisting of 0.1 mM CYP2A5 and 0.3 mM cytochrome P450 reductase, 5 mM GSH (or Gly-Cys, or Cys, or N-acetyl-Cys), and 1 mM NADPH, in a final volume of 0.1 ml. The reaction was carried out at 37°C for 30 min. In control incubations, NADPH was omitted. (A) Chromatograms of GS-DCBN, Gly-Cys-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN detected by radiometric HPLC analysis; (B) Chromatograms of GS-DCBN, Gly-Cys-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN detected by LC-MS/MS using MRM scan for m/z transitions at 459→200, 330→200, 273→156, and 315→200, respectively. cpm, counts per minute; cps, counts per second.
In vitro formation of GS-DCBN by human and mouse nasal mucosa microsomes
Previous studies using recombinant P450 proteins have shown that human CYP2A13 and CYP2A6, both expressed in the nasal mucosa, are active in catalyzing the formation of DCBN-protein adducts.5,6 However, it remained unknown whether the expression levels of CYP2A13 and CYP2A6 in human nasal mucosa are sufficiently high to support DCBN bioactivation in nasal mucosa microsomes, and how efficient human nasal mucosa microsomes are toward DCBN bioactivation in comparison with mouse nasal mucosa microsomes. Thus, we determined kinetic parameters of DCBN metabolism catalyzed by human fetal nasal microsomes, as well as by nasal mucosa microsomes of WT and Cyp2a5-null mice (Table 1). Notably, human fetal, instead of adult, nasal mucosa was studied, because adult nasal tissues were not available for this study, and because fetal nasal mucosa had been found previously to have appreciable P450 activities toward 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.18 All three microsomal samples were active in the formation of GS-DCBN. Nasal mucosa microsomes from WT mice were highly efficient in this reaction, with an apparent km of 0.43 μM and an apparent catalytic efficiency (Vmax/Km) of ~2.4 ml/min/mg protein. As expected on the basis of previous in vivo findings,4 the efficiency for GS-DCBN formation catalyzed by nasal mucosa microsomes of Cyp2a5-null mice was much reduced, by ~40 fold; the reduction was a result of large increases in apparent Km values (by ~10 fold) and decreases in apparent Vmax values (by ~4 fold), compared to WT mice. The apparent Km value for GS-DCBN formation by human fetal nasal mucosa microsomes (0.45 μM) was comparable to that of WT mouse nasal mucosa microsomes; but, the Vmax for human fetal nasal mucosa microsomes (0.14 pmol/min/mg protein) was much lower than that of mouse nasal mucosa microsomes, resulting in a very low catalytic efficiency.
Table 1.
Kinetic parameters of GS-DCBN formation catalyzed by human and mouse nasal mucosa microsomesa
| Source of nasal mucosa | Km (μM) | Vmax (pmol/min/mg protein) | Vmax/Km (μl/min/mg) |
|---|---|---|---|
| WT mouse | 0.43 ± 0.04 | 1050 ± 30 | 2440 |
| Cyp2a5-null mouse | 4.2 ± 0.6 | 253 ± 12 | 60 |
| Human fetus | 0.45 ± 0.10b | 0.14 ± 0.01 | 0.31 |
Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, DCBN at various concentrations, added in 1 μl methanol, 0.1 mg/ml (for mouse nasal mucosa) or 0.5 mg/ml (for human nasal mucosa) microsomal protein, 5 mM GSH, and 1 mM NADPH, in a final volume of 0.1 ml. The reaction was carried out at 37°C for 5 or 10 min (for mouse nasal mucosa) or 60 min (for human nasal mucosa). DCBN concentrations used were 0.05, 0.10, 0.25, 0.50, 1.0, 2.0, and 4.0 μM for WT mouse nasal mucosa; 0.50, 1.0, 2.0, 4.0, 8.0, 15, and 30 μM for Cyp2a5-null mouse nasal mucosa; and 0.10, 0.25, 0.50, 1.0, 2.0, 4.0, 8.0, and 15 μM for human nasal mucosa. Microsomal samples were prepared from pooled tissues from 10–12 mice (male and female, 2–3 month old) or from 8 human fetuses (male and female, 104–120 gestational days). Values represent means ± SE for three determinations.
P>0.05, compared to WT mouse, Student t-test
The involvement of CYP2A6/13 in DCBN bioactivation in human fetal nasal mucosa microsomes was determined with the use of 8-methoxysalen, which is a CYP2A-selective inhibitor when used at relatively low concentrations.19 We found that 8-methoxyporsalen, added (together with DCBN) at 2.5 μM, totally blocked GS-DCBN formation, with DCBN at 15 μM and human nasal mucosa microsomal proteins at 0.5 mg/ml (data not shown).
In vivo formation of GS-DCBN and its derivatives in mice
The newly developed quantitative LC-MS/MS method was employed to characterize the in vivo formation of GS-DCBN and its derivatives in DCBN-treated mice. The analytical method was validated for accuracy and precision (Table 2). Calibration curves for all analytes showed excellent linearity over the concentration range used (R2>0.99) (data not shown). The lower limit of quantification (LLOQ) was found to be 0.07 pmol (on column) for GS-DCBN, 0.01 pmol for Gly-Cys-DCBN, 0.03 pmol for Cys-DCBN, and 0.09 pmol for N-acetyl-Cys-DCBN (signal to noise ratio ≥ 5).
Table 2.
Precision and accuracy in the determination of GS-DCBN and other metabolites in serum samplesa
| Levels | GS-DCBN | Gly-Cys-DCBN | Cys-DCBN | N-acetyl-Cys-DCBN | ||||
|---|---|---|---|---|---|---|---|---|
| Spiked (nmol/ml) | 13.5 | 54 | 2.5 | 10 | 5 | 20 | 15 | 60 |
| Detected (mean) (nmol/ml) | 15.9 | 61.2 | 2.4 | 8.8 | 4.9 | 16.2 | 14.2 | 52.5 |
|
| ||||||||
| Relative error (%) | 17.8 | 13.3 | −4.4 | −12.0 | −2.0 | −19.0 | −5.3 | −12.5 |
| RSD (%) | 5.3 | 5.0 | 13.0 | 5.9 | 7.5 | 6.3 | 5.5 | 5.9 |
Precision is expressed as relative standard deviation (RSD; 100% × SD/mean). Accuracy is expressed as relative error [100% × (mean detected concentration − spiked concentration)/spiked concentration].
We first compared GS-DCBN levels in serum, nasal mucosa, and nasal wash fluid of DCBN-treated WT and Cyp2a5-null mice, at various times after a single i.p. injection of DCBN at 10 mg/kg, a dose close to the lowest reported DCBN dose (12 mg/kg, i.p.) that can induce observable pathological changes in mouse nasal mucosa.1 As shown in Figure 5A–C, GS-DCBN was readily detected in all three types of samples from DCBN-treated WT mice; in the nasal wash samples, GS-DCBN was detectable at least through the 6-h time point (Figure 5C). Notably, the maximal concentration of GS-DCBN in the nasal mucosa (~5.5 nmol/g) was much higher than that in the nasal wash fluid (~4.5 pmol/ml), indicating that only a very small fraction of the amount in the tissue was secreted to nasal mucus. In Cyp2a5-null mice, GS-DCBN levels were significantly reduced in both nasal mucosa and nasal wash fluid, although the level in serum was not significantly changed, relative to WT mice (Figure 5A–C). These results indicated not only that CYP2A5, a nasal mucosa-predominant enzyme, was a critical determinant of GS-DCBN levels in the nasal mucosa and nasal wash fluid, but also that GS-DCBN levels in nasal wash fluid were independent of GS-DCBN levels in systemic circulation.
Fig 5. GS-DCBN levels in WT and CYP2A5-null mice at various times after intraperitoneal administration of DCBN.
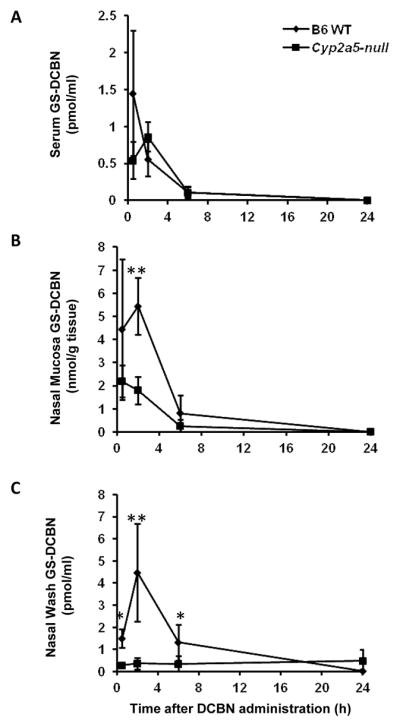
Mice (2- to 3-month old, male and female) were treated with a single i.p. injection of DCBN at 10 mg/kg. (A) Serum levels of GS-DCBN; (B) nasal mucosa levels of GS-DCBN; (C) Nasal-wash fluid levels of GS-DCBN. Data represent means ± S.D. (n = 3–6). *, P<0.05; **, P<0.01; compared with levels in WT mice.
We then performed pharmacokinetic analysis for both GS-DCBN and its metabolites in nasal wash samples, in order to identify the metabolite(s) with greatest relative abundance (AUC) and/or persistence (t1/2) in WT mice (Table 3). Mice were treated with DCBN either via a single i.p. injection (at 5 or 10 mg/kg), or via four consecutive s.c. injections (each at 1 mg/kg; 1.5-h intervals). According to the AUC values, GS-DCBN is the most abundant of the four metabolites in all three dosing protocols. However, according to the t1/2 values, Cys-DCBN and N-acetyl-Cys-DCBN are generally more persistent than are GS-DCBN and Gly-Cys-DCBN; the low t1/2 value for N-acetyl-Cys-DCBN in the 5-mg/kg group seems to be an exception. The AUC0–24 values also showed that the overall abundance of all metabolites detected in the nasal wash fluid was dependent on the DCBN dose. For the two i.p. doses, the total amount of metabolites in the 5-mg/kg group was ~50% of that in the 10-mg/kg group.
Table 3.
Pharmacokinetic parameters for GS-DCBN and other metabolites in nasal wash samples from WT micea
| DCBN dose (mg/kg), route | GS-DCBN | Gly-Cys-DCBN | Cys-DCBN | N-acetyl-Cys-DCBN | |
|---|---|---|---|---|---|
| AUC0–24 (h*pmol/ml) | 10, i.p. | 29.1 | 1.14 | 8.4 | 10.1 |
| 5, i.p. | 14.8 | 0.58 | 2.4 | 3.02 | |
| 1 × 4, s.c. | 6.2 | 0.29 | 3.4 | 3.4 | |
|
| |||||
| t1/2 (h) | 10, i.p. | 3.3 | 3.2 | 6.4 | 7.3 |
| 5, i.p. | 4.9 | 4.8 | 13.9 | 2.6 | |
| 1 × 4, s.c. | 4.9 | 3.3 | 12.5 | 11.4 | |
Two- to three-month old, male and female WT mice (n = 3–6 for each dose and time point) were treated with DCBN intraperitoneally (single injection at 5 or 10 mg/kg), or subcutaneously (four consecutive injections, each at 1 mg/kg, with 1.5-h intervals). Nasal-wash samples were collected from dissected nasal cavity of individual mice at various times (0.5–24 h) after the last dose, for LC-MS/MS analysis, as described in Materials and Methods. The mean levels of individual metabolites were used for calculation of pharmacokinetic parameters. Levels at individual time points are shown in Table 4.
An examination of the levels of individual metabolites at various times following DCBN treatment (Table 4) showed, despite large interindividual variations, interesting trends in their relative levels. Consistent with the AUC values in Table 3, GS-DCBN was generally the predominant metabolite up to 6 h after dosing, whereas Gly-Cys-DCBN was usually the least abundant. However, at 24 h after dosing, the levels of Cys-DCBN and N-acetyl-Cys-DCBN were comparable to that of GS-DCBN (for both 10-mg/kg i.p. dose groups and the s.c. injection groups), suggesting that clearance of Cys-DCBN and N-acetyl-Cys-DCBN from the nasal mucus is slower than that of GS-DCBN and Gly-Cys-DCBN.
Table 4.
Levels of GS-DCBN and other metabolites in nasal-wash samples from WT mice at various times following DCBN treatmenta
| DCBN dose (mg/kg), route | Time after injection (h) | Metabolite levels (pmol/ml)b
|
|||
|---|---|---|---|---|---|
| GS-DCBN | Gly-Cys- DCBN | Cys- DCBN | N-acetyl-Cys- DCBN | ||
| 10, i.p. | 0.5 | 1.6±0.4c | 0.05±0.03 | 0.48±0.27d | 0.57±0.11d |
| 2 | 4.5±2.2c | 0.15±0.12 | 1.0±0.96 | 1.1±0.85 | |
| 6 | 1.4±1.0c | 0.06±0.06 | 0.42±0.32 | 0.52±0.29 | |
| 24 | 0.04±0.05 | 0.001±0.002 | 0.08±0.05d | 0.12±0.09d | |
|
| |||||
| 5, i.p. | 0.5 | 0.53±0.18e | 0.03±0.02 | 0.17±0.06 | 0.37±0.16e |
| 2 | 1.6±0.74c | 0.06±0.03 | 0.19±0.10 | 0.42±0.15 | |
| 6 | 0.91±0.87c | 0.03±0.02 | 0.16±0.16 | 0.14±0.13 | |
| 24 | <0.2 | <0.03 | <0.09 | <0.28 | |
|
| |||||
| 1 × 4, s.c. | 0.5 | 1.4±0.9 | 0.11±0.08f | 0.39±0.20 | 0.36±0.10 |
| 2 | 0.44±0.10 | 0.03±0.01f | 0.12±0.10 | 0.31±0.50 | |
| 6 | 0.33±0.20 | 0.01±0.00f | 0.20±0.02 | 0.14±0.10 | |
| 24 | 0.03±0.03 | <0.03 | 0.07±0.03 | 0.08±0.10 | |
Experimental design is described in the legend to Table 3.
Values represent means ± S.D. (n = 3–6 for each time point). Statistical significance of differences in levels of the four metabolites at each time point was analyzed using One-way analysis of variance followed by Student-Newman-Keuls test.
P<0.05, compared to all other metabolites
P<0.05, compared to Gly-Cys-DCBN levels
P<0.05, compared to Cys-DCBN and Gly-Cys-DCBN levels
P<0.05, compared to GS-DCBN levels
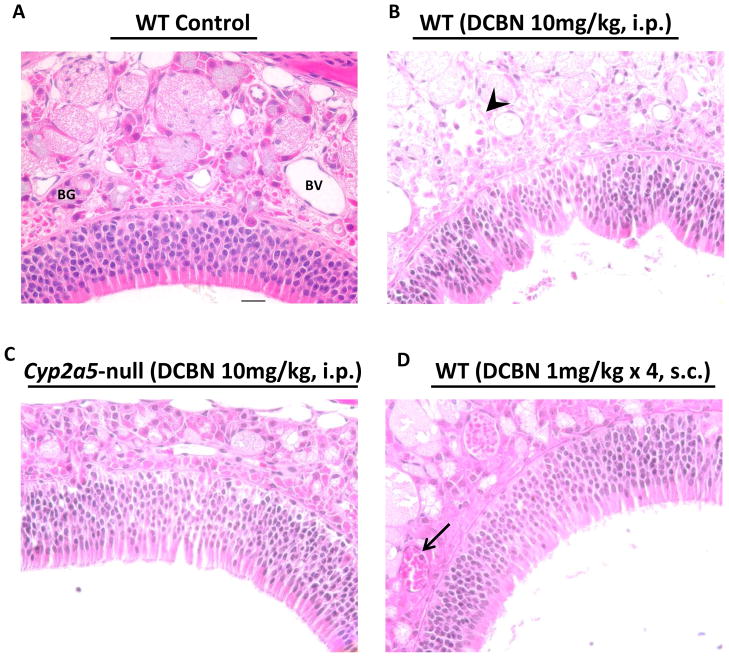
The dose-dependent increase in amounts of GS-DCBN and/or its further metabolites detected in the nasal wash samples paralleled the dose-dependent occurrence of DCBN-induced damage in the nasal mucosa. As shown in Figure 6, at 24 h after a single i.p. injection of DCBN at 10 mg/kg, WT mice showed typical pathological changes in the nasal mucosa, including the fragmentation of Bowman’s glands in the submucosa and deformation of the neuroepithelium (Figure 6B); these signs of toxicity were concordant with the detection of abundant GS-DCBN in both nasal mucosa and nasal wash samples (Figure 5B, C). In Cyp2a5-null mice treated with DCBN at 10 mg/kg, both the epithelium and the submucosa were intact at 24 h after dosing (Figure 6C), consistent with the much decreased levels of GS-DCBN in nasal mucosa (Figure 5B), and the near absence of GS-DCBN in the nasal wash fluid (Figure 5C). In WT mice treated with multiple s.c. injections of DCBN at 1 mg/kg, appreciable amounts of GS-DCBN (and other metabolites) were detected in the nasal wash fluid (Table 3, 4), indicating occurrence of in vivo DCBN bioactivation in the nasal mucosa, even at the very low dose administered. However, the AUC value for nasal wash GS-DCBN levels was ~5 times lower in the s.c. group than in the 10-mg/kg i.p. group (Table 3). Consistently, the nasal mucosa of WT mice treated with the s.c. injection protocol showed no evident pathologic changes at 24 h after the last injection (Figure 6D), except for a hint of mild inflammation, as suggested by aggregation of red blood cells in some blood vessels and limited infiltration of neutrophils.
Fig 6. Histopathological analysis of nasal mucosal damages caused by DCBN treatment in WT and CYP2A5-null mice.
Two- to three-month old mice were treated with a single i.p. injection of DCBN (at 10 mg/kg), or four consecutive subcutaneous injections of DCBN (at 1 mg/kg, and with 1.5-h intervals), or with the vehicle alone. Tissues were obtained for histopathological examination at 24 h after the last treatment. Representative H&E-stained cross sections of the dorsal medial region of the nasal cavity, obtained at the level 3,36 are shown. (A) Vehicle-treated mice had normal lamina propria with intact Bowman’s glands (BG) and clear blood vessels (BV). The lamina propria is covered by a pseudostratified olfactory neuroepithelium of normal thickness. (B) DCBN-treated WT mice (10 mg/kg, i.p.) showed moderate injury to the nasal mucosa, evident by deformed epithelium still attached to the basement membrane, and degenerated BG in the lamina propria (arrowhead). (C) DCBN-treated Cyp2a5-null mice (10 mg/kg, i.p.) showed essentially normal olfactory epithelium and BG. (D) DCBN-treated WT mice (4 × 1 mg/kg, s.c.) showed essentially normal morphology, except for aggregation of red blood cells in BV and occasional presence of neutrophils (arrow). Scale bar: 20 μm.
Discussion
As an herbicide commonly used to control weeds in gardens and lawns and various other settings, DCBN can be contacted by humans in a range of scenarios, e.g., exposure by workers who prepare or apply the herbicide, by members of the public who travel in treated vegetation or consume drift-contaminated berries or vegetables.20 The relatively strong persistence of DCBN leads to its widespread occurrence in the environment, including air, soil, ground water, river, and sea,21 which increases the chance of human exposure. A recent study showed that DCBN in herbicides can permeate through both disposable and some chemically protective nitrile gloves,22 further suggesting the risks of DCBN exposure by workers.
The U.S. Environmental Protection Agency (EPA) classifies herbicides containing DCBN (e.g. NOROSAC and Casoron) as toxicity class III (low toxicity), with a signal word “CAUTION,” based on animal experiments; however, the study did not assess nasal toxicity.23 There have been no reported biomonitoring studies on the levels of DCBN in people or epidemiological studies examining the health effects of environmental exposure to DCBN on humans. Thus, although the potent and nasal-specific toxicity of DCBN in rodents has been studied extensively,1,7,24,25,26 available information for extrapolating these results to human beings is very limited.
Using the analytical method established in the current study, we have obtained the first evidence that human fetal nasal tissue is capable of metabolizing DCBN to reactive intermediates, a result supporting potential risks of DCBN toxicity in human nose. The fact that mouse and human nasal mucosa microsomes had similar Km values toward DCBN bioactivation implies that human nasal mucosa is active toward DCBN in the same DCBN concentration range as is mouse nasal mucosa. The low rate of GS-DCBN formation catalyzed by human fetal nasal microsomes is most likely due to the low levels of P450 present in the fetal tissue samples. It is expected that, in healthy human adults, the nasal P450 expression levels will be higher, thus supporting greater activity toward DCBN. Age-related increases (fetal through adult) in irreversible binding of radio-labeled DCBN to the nasal mucosa, and in DCBN-induced nasal toxicity, were previously found in mice.27 In humans, although levels of CYP2A13 protein were not found to be higher in adult biopsy tissues than in fetal nasal mucosa in a previous study,14 the adult nasal tissues used were from patients, not from healthy individuals, for whom a higher level of CYP2A13 is expected. In addition, the CYP2A proteins are known to be expressed in cell- and zone-specific manners in nasal tissues in both rodents and humans.14,28 The greater abundance of CYP2A proteins in nasal microsomes from rodents than that from humans is largely due to the much larger olfactory regions, where cells with high CYP2A expression reside, in rodents than in humans. Therefore, the rates of DCBN bioactivation in CYP2A-expressing cells in the olfactory region are much more comparable between mice and humans than are the rates determined for microsomes prepared from the whole nasal mucosa; this fact, combined with the low Km of the CYP2A enzymes in GS-DCBN formation in nasal mucosa microsomes, suggest that, even at a low exposure levels, DCBN has the potential to induce focal lesions in human nasal mucosa, through selective damage to those cells expressing higher levels CYP2A.
Experimental evidence for occurrence of in vivo DCBN bioactivation in human nasal tissue remains to be obtained. Such evidence, which is critical for risk assessment, may be obtained through detection of DCBN metabolites in exposed individuals. Based on risk assessment conducted by U.S. EPA,29 the average daily exposure to DCBN by applicators is 0.024 to 0.034 mg/kg bw/day through dermal absorption, and 0.00411 to 0.00583 mg/kg bw/day through inhalation. It can thus be estimated that, for an applicator of 70 kg in weight, a maximum of ~0.3 mg (or ~2 μmol) DCBN could be absorbed through inhalation, each day. It has been shown that >10% of the inhaled dose are deposited in the nasal tissue following a nose-only aerosol exposure in rats.30 If we assume similar nasal dosimetry in rats and humans, >0.2 μmol of DCBN would be expected to be deposited in the applicator’s nasal tissue each day, which would yield a maximal DCBN concentration of >0.5 mM in the nasal tissue, presumably at a weight of ~0.4 g and of the same density as water,31 in the absence of clearance. The actual intracellular concentration at any given time during exposure will depend on many factors, in addition to the rate of clearance; however, it seems likely that the peak levels will be greater than the Km determined here for GS-DCBN formation (0.45 μM) by human fetal nasal microsomes. Therefore, it can be expected that DCBN absorbed through inhalation exposure will be efficiently bioactivated in the applicators’ nasal tissue, especially in those individuals, or in nasal regions, with relatively high expression of CYP2A13.
In the present animal study, we have established sensitive methods for detection of DCBN metabolites in nasal wash fluid; confirmed the relevance of the levels of these metabolites in nasal wash fluid to DCBN bioactivation and toxicity in the nasal mucosa; and demonstrated the relative persistence of these metabolites in nasal-wash fluid. Our results suggest that in the scenarios of human exposure to DCBN, the various metabolites, particularly GS-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN, in nasal-wash fluids are useful for bio-monitoring of DCBN exposure as well as its potential nasal toxicity. In that regard, whereas the combined levels of GS-DCBN and its further metabolites may represent the extent of DCBN exposure and bioactivation in the nasal mucosa, the relative amounts of GS-DCBN to its metabolites in nasal-wash fluids may reflect the length of time between the last exposure and the collection of nasal-wash sample.
For biomonitoring of DCBN exposure in human population, GS-DCBN and its metabolites in nasal-wash fluids appear to satisfy most conditions required as biomarkers,32 including (1) “specifically assesses the exposure to the chemical substance under investigation” (GS-DCBN and its metabolites could not be generated from compounds other than DCBN), (2) “be sufficiently sensitive to detect subjects exposed to low levels of chemicals” [GS-DCBN and its metabolites could be detected in mouse nasal-wash fluids even when mice were exposed to non-toxic doses of DCBN; while the ability of our method to detect DCBN metabolites in human nasal wash remains to be demonstrated, at least two factors pertinent to human DCBN exposure would serve to increase sensitivity of detection, including the chronic/continuous nature of exposure by applicators (usually within a 3-month period), and the fact that humans produce much more nasal mucus (~0.1 ml/min33) than mice do (only ~3 μl mucus could be collected from the nasal cavity34)], (3) “vary quantitatively with the intensity of exposure and/or the risk of adverse effects” (as indicated by results obtained at different DCBN doses), (4) “yield more information on potential health risks than ambient monitoring” (metabolites are derived from reactive intermediates, and their formation correlated with occurrence of toxicity), (5) “be stable enough to allow storage of the sample for a certain period of time” (the nasal-wash fluids were stable when stored at −80°C for at least several months), (6) “not to entail too much discomfort or any health risk for the subject” (collection of nasal flush samples is non-invasive and can be easily performed in humans), (7) “be measured by an analytical method presenting sufficient accuracy, specificity and sensitivity” (the LC-MS assay fits all three requirements; further increases in sensitivity are possible by using newer generation of instrument). A shortcoming of using GSH-conjugates as biomarkers, compared to DNA- or protein-adducts, is their relatively short biological half-life. Therefore, GS-DCBN and its metabolites are only appropriate for monitoring recent exposures. Notably, given the ability of DCBN to cause permanent loss of olfactory stem cells,4 early detection of DCBN exposure and in vivo bioactivation may provide enough time for therapeutic intervention. Additionally, it may be difficult to predict, via reverse dosimetry, the level of DCBN exposure based on detected GSH-conjugates levels, given the added complexity by the involvement of multiple metabolic steps.
Urinary mercapturic acids have traditionally been suggested as useful markers for human biological monitoring studies;35 however, their utility as biomarkers of extrahepatic tissue xenobiotic toxicity is questionable, particularly for small organs such as the nose and for extrahepatic, tissue-specific toxicants, such as DCBN. In this study, we obtained evidence that GS-DCBN and its metabolites in nasal wash fluids are markers of DCBN nasal toxicity, as well as extents of DCBN exposure at the target tissue. Through the use of the Cyp2a5-null mouse model, we showed that the levels of GS-DCBN in nasal-wash samples were independent of its systemic circulating level, but dependent on the presence of CYP2A5, a nasal mucosa-predominant P450 enzyme that is highly active in DCBN bioactivation. This result is consistent with our previous finding that nasal toxic intermediates of DCBN are produced via local bioactivation.4 Furthermore, GS-DCBN and its metabolites were detected in the nasal-wash fluid regardless of whether DCBN was injected intraperitoneally or subcutaneously; the latter injection regimen not only reduces (by avoiding first-pass metabolism) the amounts of circulating DCBN metabolites that are formed by the liver, but also mimics repeated low-dose human exposure to DCBN in an occupational setting through skin absorption. Therefore, biomonitoring of GS-DCBN and its further metabolites in nasal-wash fluid can provide relevant information about potential risks of DCBN nasal toxicity in an individual; whereas urinary metabolites, including the GSH-conjugates and derivatives, as well as other DCBN metabolites, such as hydroxy DCBN, would only be suitable as markers for systemic exposure to DCBN.
In summary, findings from this study provide not only analytical methods, but also scientific basis for the potential utility of GS-DCBN and its further metabolites in nasal-wash fluid as biomarkers of exposure as well as nasal toxicity of DCBN, a commonly used herbicide and tissue-specific toxicant, in human populations. The methods and approaches established here might also be applied to biomarker studies on other nasal toxic drugs or contaminants that exert toxicity through thiol-reactive intermediates.
Acknowledgments
We gratefully acknowledge the use of the services of the Pathology Core and the Advanced Light Microscopy and Image Analysis Core Facilities of the Wadsworth Center. We thank Dr. David Spink of the Wadsworth Center for advice on the preparation of DCBN metabolite standards, and Ms. Weizhu Yang for assistance with mouse breeding. This study was supported in part by grant ES007462 from the National Institute of Environmental Health Sciences, NIH.
Abbreviations
- DCBN
2,6-dichlorobenzonitrile (or dichlobenil)
- P450 or CYP
cytochrome P450
- GSH
reduced glutathione
- HPLC
high-performance liquid chromatography
- MS
mass spectrometry
- NL
neutral loss
- EPI
enhanced product ion
- AUC
area under the concentration-time curve
- WT
wild-type
- GS-DCBN
glutathione conjugate of DCBN
- Gly-Cys
γ-L-glutamyl-L-cysteine
- N-acetyl-Cys
N-acetyl-L-cysteine
References
- 1.Brandt I, Brittebo EB, Feil VJ, Bakke JE. Irreversible binding and toxicity of the herbicide dichlobenil (2,6-dichlorobenzonitrile) in the olfactory mucosa of mice. Toxicol Appl Pharmacol. 1990;103:491–501. doi: 10.1016/0041-008x(90)90322-l. [DOI] [PubMed] [Google Scholar]
- 2.Bergman U, Ostergren A, Gustafson AL, Brittebo B. Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol. 2002;76:104–112. doi: 10.1007/s00204-002-0321-2. [DOI] [PubMed] [Google Scholar]
- 3.Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Xie F, Zhou X, Behr M, Fang C, Horii Y, Gu J, Kannan K, Ding X. Mechanisms of olfactory toxicity of the herbicide 2,6-dichlorobenzonitrile: essential roles of CYP2A5 and target-tissue metabolic activation. Toxicol Appl Pharmacol. 2010;249:101–106. doi: 10.1016/j.taap.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Zhuo X, Gonzalez FJ, Ding X. Baculovirus-mediated expression and characterization of rat CYP2A3 and human CYP2a6: role in metabolic activation of nasal toxicants. Mol Pharmacol. 1996;50:781–788. [PubMed] [Google Scholar]
- 6.Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- 7.Eriksson C, Brittebo EB. Metabolic activation of the olfactory toxicant, dichlobenil, in rat olfactory microsomes: comparative studies with p-nitrophenol. Chem Biol Interact. 1995;94:183–196. doi: 10.1016/0009-2797(94)03333-4. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Spink DC, Bhama JK, Sheng JJ, Vaz AD, Coon MJ. Metabolic activation of 2,6-dichlorobenzonitrile, an olfactory-specific toxicant, by rat, rabbit, and human cytochromes P450. Mol Pharmacol. 1996;49:1113–1121. [PubMed] [Google Scholar]
- 9.Brittebo EB, Eriksson C, Brandt I. Effects of glutathione-modulating agents on the covalent binding and toxicity of dichlobenil in the mouse olfactory mucosa. Toxicol Appl Pharmacol. 1992;114:31–40. doi: 10.1016/0041-008x(92)90093-8. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Wen B, Ruan Q, Zhu M. Rapid screening of glutathione-trapped reactive metabolites by linear ion trap mass spectrometry with isotope pattern-dependent scanning and postacquisition data mining. Chem Res Toxicol. 2008;21:1477–1483. doi: 10.1021/tx8001115. [DOI] [PubMed] [Google Scholar]
- 11.van Welie RT, van Dijck RG, Vermeulen NP, van Sittert NJ. Mercapturic acids, protein adducts, and DNA adducts as biomarkers of electrophilic chemicals. Crit Rev Toxicol. 1992;22:271–306. doi: 10.3109/10408449209146310. [DOI] [PubMed] [Google Scholar]
- 12.Gu J, Zhang QY, Genter MB, Lipinskas TW, Negishi M, Nebert DW, Ding X. Purification and characterization of heterologously expressed mouse CYP2A5 and CYP2G1: role in metabolic activation of acetaminophen and 2,6-dichlorobenzonitrile in mouse olfactory mucosal microsomes. J Pharmacol Exp Ther. 1998;285:1287–1295. [PubMed] [Google Scholar]
- 13.Gu J, Walker VE, Lipinskas TW, Walker DM, Ding X. Intraperitoneal administration of coumarin causes tissue-selective depletion of cytochromes P450 and cytotoxicity in the olfactory mucosa. Toxicol Appl Pharmacol. 1997;146:134–143. doi: 10.1006/taap.1997.8238. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Liu YQ, Su T, Ren X, Shi L, Liu D, Gu J, Zhang QY, Ding X. Immunoblot analysis and immunohistochemical characterization of CYP2A expression in human olfactory mucosa. Biochem Pharmacol. 2003;66:1245–1251. doi: 10.1016/s0006-2952(03)00476-3. [DOI] [PubMed] [Google Scholar]
- 15.Wen B, Ma L, Zhu M. Bioactivation of the tricyclic antidepressant amitriptyline and its metabolite nortriptyline to arene oxide intermediates in human liver microsomes and recombinant P450s. Chem Biol Interact. 2008;173:59–67. doi: 10.1016/j.cbi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Hou Y, Jiao X, Gu XX. Intranasal immunization with a lipooligosaccharide-based conjugate vaccine from nontypeable Haemophilus influenzae enhances bacterial clearance in mouse nasopharynx. FEMS Immunol Med Microbiol. 2003;35:1–10. doi: 10.1111/j.1574-695X.2003.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Wong HL, Zhang X, Zhang QY, Gu J, Ding X, Hecht SS, Murphy SE. Metabolic activation of the tobacco carcinogen 4-(methylnitrosamino)-(3-pyridyl)-1-butanone by cytochrome P450 2A13 in human fetal nasal microsomes. Chem Res Toxicol. 2005;6:913–918. doi: 10.1021/tx0500777. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, D’Agostino J, Wu H, Zhang Q-Y, von Weymarn L, Murphy SE, Ding X. CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther. 2007;323:570–578. doi: 10.1124/jpet.107.127068. [DOI] [PubMed] [Google Scholar]
- 20.Washington State Department of Transportation. Roadside vegetation management herbicide fact sheet: dichlobenil. 2006 http://www.wsdot.wa.gov/NR/rdonlyres/37DC03D2-3E93-4A36-AF6A-F08066A3A6BB/0/dichlobenil.pdf.
- 21.Björklund E, Styrishave B, Anskjœr GG, Hansen M, Halling-Sørense B. Dichlobenil and 2,6-dichlorobenzamide (BAM) in the environment: What are the risks to humans and biota? Sci Total Environ. 2011;19:3732–3739. doi: 10.1016/j.scitotenv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Que Hee SS, Zainal H. Permeation of herbicidal dichlobenil from a Casoron formulation through nitrile gloves. Arch Environ Contam Toxicol. 2010;58:249–254. doi: 10.1007/s00244-009-9407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Environmental Protection Agency. Prevention, pesticides and toxic substances (7508C) EPA-738-F-98-005. RED Facts Dichlobenil. 1998 Oct; http://www.epa.gov/oppsrrd1/REDs/factsheets/0263fact.pdf.
- 24.Brittebo EB, Eriksson C, Feil V, Bakke J, Brandt I. Toxicity of 2,6-dichlorothiobenzamide (chlorthiamid) and 2,6-dichlorobenzamide in the olfactory nasal mucosa of mice. Fundam Appl Toxicol. 1991;17:92–102. doi: 10.1016/0272-0590(91)90242-v. [DOI] [PubMed] [Google Scholar]
- 25.Deamer NJ, O’Callaghan JP, Genter MB. Olfactory toxicity resulting from dermal application of 2,6-dichlorobenzonitrile (dichlobenil) in the C57Bl mouse. Neurotoxicology. 1994;15:287–293. [PubMed] [Google Scholar]
- 26.Genter MB, Owens DM, Carlone HB, Crofton KM. Characterization of olfactory deficits in the rat following administration of 2,6-dichlorobenzonitrile (dichlobenil), 3,3′-iminodipropionitrile, or methimazole. Fundam Appl Toxicol. 1996;29:71–77. doi: 10.1006/faat.1996.0007. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson C, Brittebo EB. Dichlobenil in the fetal and neonatal mouse olfactory mucosa. Toxicology. 1995;96:93–104. doi: 10.1016/0300-483x(94)02914-g. [DOI] [PubMed] [Google Scholar]
- 28.Piras E, Franzén A, Fernández EL, Bergström U, Raffalli-Mathieu F, Lang M, Brittebo EB. Cell-specific expression of CYP2A5 in the mouse respiratory tract: effects of olfactory toxicants. J Histochem Cytochem. 2003;51:1545–1555. doi: 10.1177/002215540305101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Environmental Protection Agency, Washington, D.C. 20460, Office of Prevention, Pesticides and Toxic Substances. Memorandum. Jun 11, 2008. Dichlobenil; Human Health Risk Assessment for Proposed Uses on Rhubarb; Caneberry, Subgroup 13-07A; and Bushberry, Subgroup 13-07B. EPA-HQ-OPP-2007-0604-0004.pdf. [Google Scholar]
- 30.Sakagami M, Kinoshita W, Sakon K, Makino Y. Fractional contribution of lung, nasal and gastrointestinal absorption to the systemic level following nose-only aerosol exposure in rats: a case study of 3.7-μm fluorescein aerosols. Arch Toxicol. 2003;77:321–329. doi: 10.1007/s00204-003-0450-2. [DOI] [PubMed] [Google Scholar]
- 31.Bogdanffy MS, Sarangapani R, Kimbell JS, Frame SR, Plowchalk DR. Analysis of vinyl acetate metabolism in rat and human nasal tissues by an in vitro gas uptake technique. Toxicol Sci. 1998;46:235–246. doi: 10.1006/toxs.1998.2542. [DOI] [PubMed] [Google Scholar]
- 32.Hoet P, Haufroid V. Biological monitoring: state of the art. Occup Environ Med. 1997;54:361–366. doi: 10.1136/oem.54.6.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor DF, Andersen I, Lundqvist GR. Human nasal mucosal function at controlled temperatures. Respir Physiol. 1977;30:109–124. doi: 10.1016/0034-5687(77)90025-1. [DOI] [PubMed] [Google Scholar]
- 34.Ito R, Ozaki YA, Yoshikawa T, Hasegawa H, Sato Y, Suzuki Y, Inoue R, Morishima T, Kondo N, Sata T, Kurata T, Tamura S. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362–2371. doi: 10.1016/s0264-410x(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen NP. Analysis of mercapturic acids as a tool in biotransformation, biomonitoring and toxicological studies. Trends Pharmacol Sci. 1989;10:177–181. doi: 10.1016/0165-6147(89)90232-0. [DOI] [PubMed] [Google Scholar]
- 36.Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]