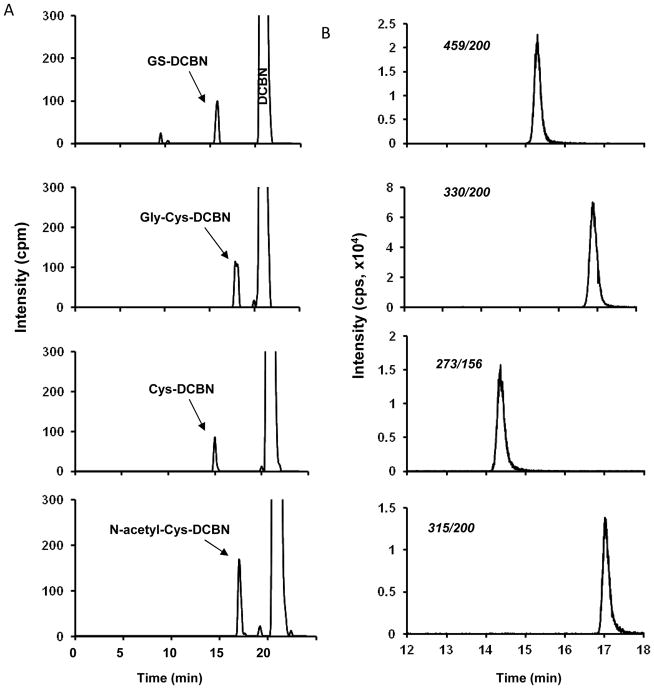
Fig 4. Enzymatic synthesis and quantification of metabolite standards for GS-DCBN and its derivatives.
Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 1 μM DCBN (including 2,6-[ring-14C]DCBN, 20.1 Ci/mol, for samples to be analyzed by radiometric LC), added in 1 μl of methanol, a reconstituted P450 system consisting of 0.1 mM CYP2A5 and 0.3 mM cytochrome P450 reductase, 5 mM GSH (or Gly-Cys, or Cys, or N-acetyl-Cys), and 1 mM NADPH, in a final volume of 0.1 ml. The reaction was carried out at 37°C for 30 min. In control incubations, NADPH was omitted. (A) Chromatograms of GS-DCBN, Gly-Cys-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN detected by radiometric HPLC analysis; (B) Chromatograms of GS-DCBN, Gly-Cys-DCBN, Cys-DCBN, and N-acetyl-Cys-DCBN detected by LC-MS/MS using MRM scan for m/z transitions at 459→200, 330→200, 273→156, and 315→200, respectively. cpm, counts per minute; cps, counts per second.