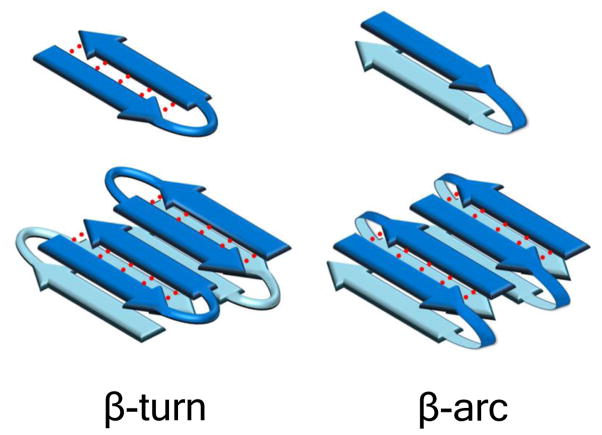
Figure 1.
Reverse turn models of polyQ peptides and their amyloid fibrils. Schematic anti-parallel β-sheet models for a segment of amyloid fibril showing two fundamental ways that fibrils might accommodate longer peptides requiring reverse turns for optimal involvement in fibril structure. In the left panel, monomers are folded into β-hairpins mediated by β-turn chain reversals. In the right panel, monomers are folded into β-arches mediated by β-arc chain reversals. Red dotted lines denote backbone intra-strand H-bonding.