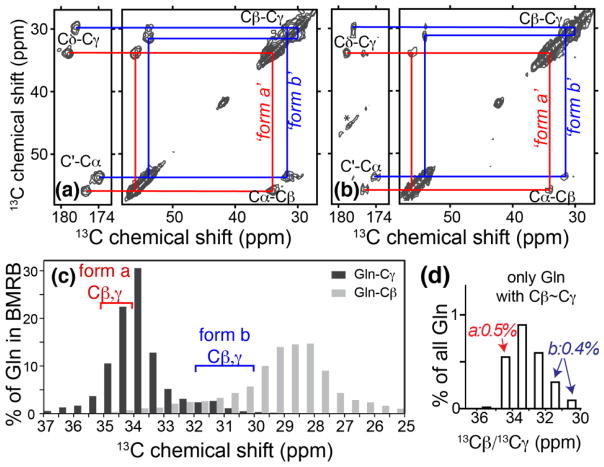
Figure 7.
Solid-state NMR on fibrils. (a) Doubled cross-peak patterns of a single labeled Gln in fibrils prepared from [U-13C, 15N-Q10] K2Q11PGQ11D2 reproduce the Gln peaks in fibrils (b) from [U-13C, 15N-Q6] K2Q30K2 peptide that lacks β-hairpin encouraging motifs 15. Red and blue lines mark peaks of the two distinct conformers. Asterisks mark spinning side bands. Spectra were obtained at 600MHz (for 1H), using 10 kHz MAS and 8ms or 25ms DARR mixing, respectively. (c) For the >10,000 Gln in the Biomolecular NMR database (bar graphs) the Gln Cβ (light bars) and Cγ shifts (dark bars) are generally well-separated. In each of the two polyQ conformers ‘form a’ (red bracket) and ‘form b’ (blue) there is instead small shift difference between Cβ and Cγ. The Cβ shift of ‘form a’ is highly unusual, and the Cβ and Cγ shifts of ‘form b’ are both atypical. (d) The average shifts of the few BMRB Gln that feature Cβ/Cγ shifts very close together (within 1 ppm). The particular shifts of conformer a (red) or b (blue) are seen in 0.5% and 0.4% of all Gln, respectively.