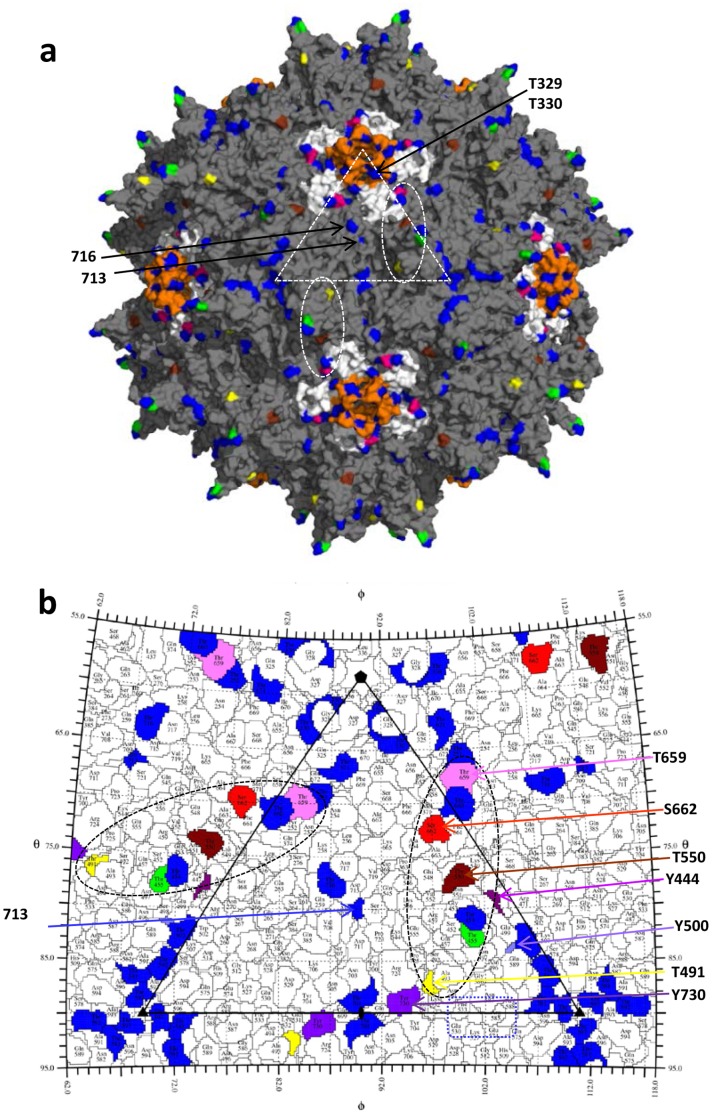
Figure 7. The AAV2 capsid surface.
(a) The capsid surface of AAV2 (grey) with the 17 surface threonine residues mutated in blue (251, 329, 330, 454, 503, 581, 592, 597, 660, 671, 701, 713, 716), green (455), yellow (491), brown (550), and pink (659). The surface location of T329, T330, T713 and T716 are indicated by arrows. The five-fold symmetry related DE loops (between the βD and βE strands) are colored in orange. The HI loops (between the βH and βI strands) are colored white and S662 located in this loop is in red. The white dashed triangle in (a) depicts a viral asymmetric unit bounded by a five-fold axis and two three-fold axes with a two-fold axis between the three-folds. Dashed ovals delineate the approximate footprints (2/60) of threonine residues that affect transduction when mutated. (b) A “Roadmap” projection [23] of the AAV2 capsid surface residues within a viral asymmetric unit. The areas covered by AAV2 surface threonines and S662 are colored as in (a). The residues in the tyrosine triple mutant residues, 444, 500, and 730 are shown in shades of purple. Dashed ovals are as described in (a). Dashed rectangle (blue) shows residues previously determined to be important in heparin sulfate receptor binding for AAV2 and AAV6 [37], [50].