Fig. 5.
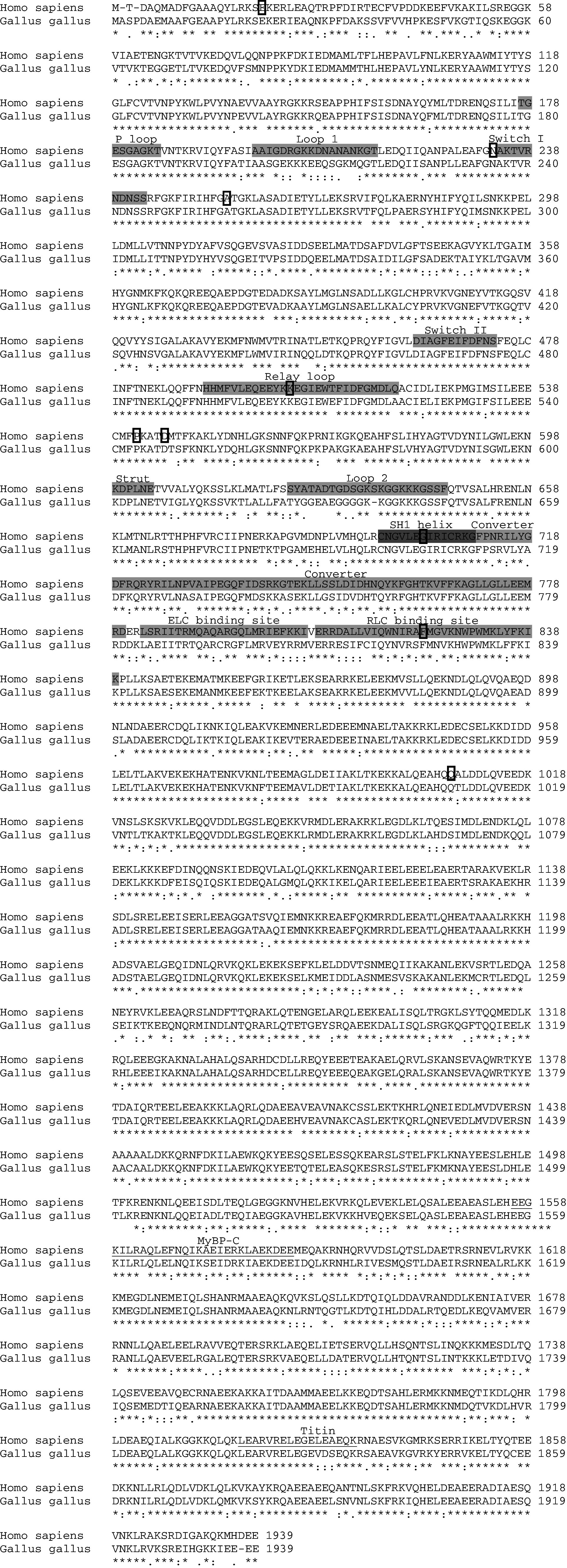
Comparison of human and chick αMHC protein sequences. The human αMHC protein sequence (NP_002462) is compared to the chick sequence (NP_001013415), with various structural domains denoted on the human sequence [158]. The sequences were aligned in ClustalW2 [159, 160]. The nucleotide (ATP)-binding pocket is in part composed of P loop, Loop I, and Switch I with Switch II also important in its function. The rigid relay loop is proposed to connect the ATP binding site to the converter domain. The Strut and Loop 2 are regions that bind the upper and lower 50-kDa subdomains. Switch II is thought to be important in forming a kink, and allowing movement of the converter domain. The converter domain is a socket for the carboxy terminal helical tail and is where rotation occurs around the SH1 helix (also termed the “fulcrum” within the literature), allowing bending of the molecule. The proposed domains for the binding of titin and myosin binding protein-C (MyBP-C) are also denoted (underlined) [161, 162]. ELC essential light chain, RLC regulatory light chain, asterisk fully conserved residues, colon residues with strongly similar properties conserved; period residues with weakly similar properties conserved. Missense mutations previously described in the human MYH6 gene [2, 58, 59] and listed in Table 2 are denoted (boxed)
