Fig. 1.
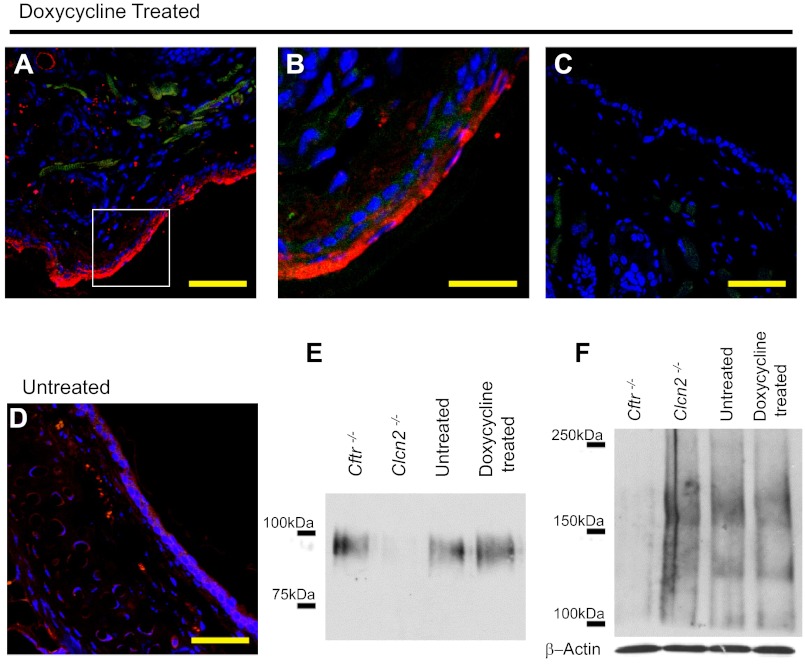
Doxycycline-treated TGN-CLCN2 mice show elevated CLCN2 expression. Expression of CLCN2 in doxycycline-treated mouse nasal section (representative of duplicate). Doxycycline-treated or untreated mouse nasal cavities were handled as described in methods. Nasal sections were stained with antibodies to CLCN2 (red), actin (green) to outline cell structure, and DAPI for nuclei (blue). A: immunostained confocal microscopy image shows CLCN2 expression in doxycycline-treated nasal cavity. B: high magnification of image in A shows CLCN2 (arrow) is expressed along the apical region of the epithelia. C: no primary antibody-treated tissue section to demonstrate absence of CLCN2 signal as a negative control. D: native untreated tissue shows reduced CLCN2 expression. Scale bar = 50 μm (A, C, D), 20 μm (B). For E and F, nasal epithelial tissue was excised from Cftr−/−, Clcn2−/−, doxycycline-treated TGN-CLCN2, and untreated TGN-CLCN2 mice. The same samples were equalized for total protein and used to determine both CLCN2 (E) and CFTR (F) expression. E: CLCN2 immunoblot of nasal tissue dissected from Cftr−/−, Clcn2−/−, doxycycline-treated, and untreated mice. CLCN2 was immunoprecipitated and separated on a 6% SDS PAGE gel. After transfer, the membrane was probed for CLCN2 as described in methods. CLCN2 (97 kDa) expression increased following induction, is considerable in the Cftr−/− mouse, and is absent in the Clcn2−/− mouse. F: CFTR immunoblot of nasal whole tissue lysate. CFTR expression (immature B-band ∼135 kDa, mature C-band ∼170 kDa) is detectable in the Clcn2−/−, untreated TGN-CLCN2, and doxycyline-treated TGN-CLCN2 mice, and absent in the Cftr−/− mouse. Equal sample loading in each lane was confirmed by an independent immunoblot for β-actin.