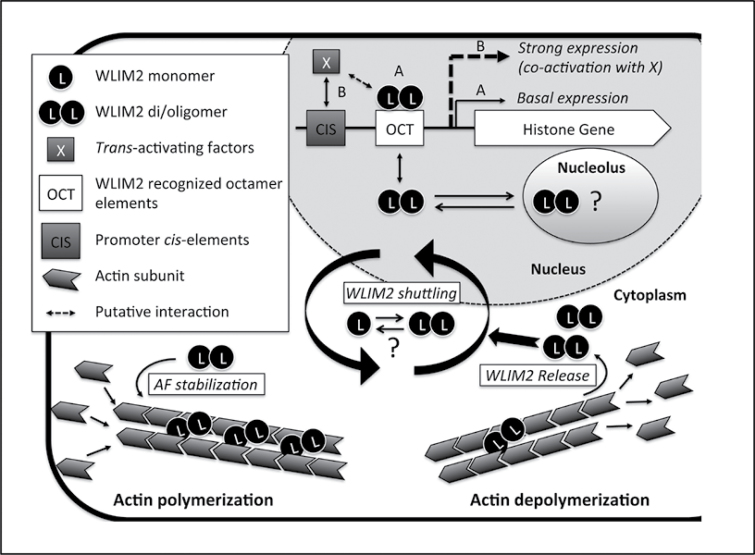
Figure 10.
Model of NtWLIM2 Functions and Cytoplasmic–Nuclear Shuttling. In the cytoplasm, NtWLIM2 (WLIM2) directly interacts with actin filaments, promotes the formation of actin bundles, and thereby contributes to stabilize the actin cytoskeleton. In the nucleus, WLIM2 interacts with Oct(II) and dOct histone promoter elements (OCT) and activates basal histone gene transcription (pathway A). Modulation of histone transcription levels would require additional trans-activating factors (X) able to target their corresponding cis-elements (CIS) in response to specific conditions (pathway B). In addition, WLIM2 might be further activated by interacting with co-factors (dashed double arrow) and/or by posttranslational modifications. Finally, both GFP-fused and endogenous forms of WLIM2 localize in the nucleolus, suggesting that WLIM2 participates in rDNA and/or ribosomal gene regulation as well. There are several lines of evidence that NtWLIM2 di/oligomerizes in the different subcellular compartments, although the significance of this process remains to be addressed. The exact mechanism underlying WLIM2 cytoplasmic–nuclear shuttling also requires further investigation. However, our data support that the intracellular distribution of NtWLIM2 is influenced by the cellular actin polymerization status. Upon actin filament depolymerization (e.g. by latrunculin B), WLIM2 is released in the cytoplasm and subsequently accumulates in the nucleus. Based on the present study and recent data obtained for the mammalian counterparts of plant LIMs, namely the cysteine-rich proteins (Kihara et al., 2011), we propose that the two LIM domain-containing proteins function as cytoskeletal stabilizers able to sense and transmit signals from actin filaments to the nucleus, where they modulate gene expression.