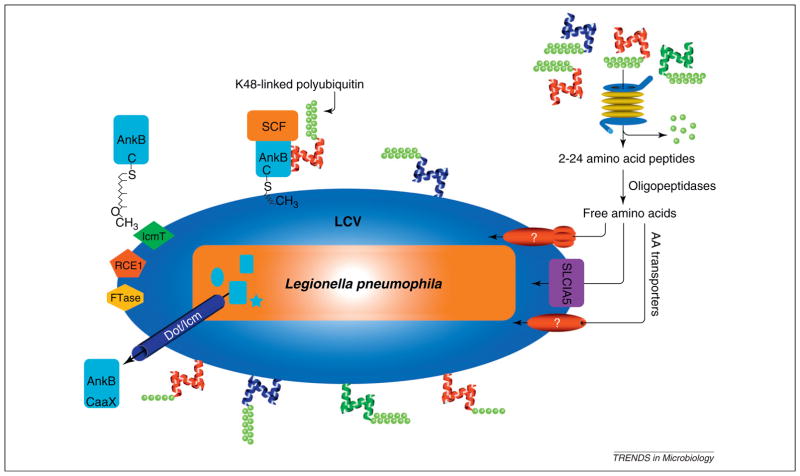
Figure 2.
Ankyrin B (AnkB)-mediated generation of amino acids through promoting proteasomal degradation of amoeba and mammalian hosts. Upon translocation of AnkB by the defect in organelle trafficking/intracellular multiplication (Dot/Icm) type IV secretion system, AnkB is farnesylated by the three host enzymes farnesyl transferase (FTase), Ras-converting enzyme-1 (RCE1) and isoprenyl cysteine carboxyl methyl transferase (IcmT) that are recruited to the Legionella-containing vacuole (LCV) by the Dot/Icm system. Upon farnesylation, AnkB is anchored into the cytosolic face of the LCV membrane, where it interacts with the SCF1 E3 ubiquitin ligase complex and acts as a platform for the docking of K48-linked polyubiquitinated proteins to the LCV. The K48-linked polyubiquitinated proteins are targeted for proteasomal degradation that generates short peptides that are rapidly degraded by cytosolic oligo- and amino-peptidases. This results in generation of high levels of free amino acids that are imported to the LCV by various amino acid (AA) transporters, such as SLC1A5. The whole process is highly conserved and is essential for intracellular proliferation of Legionella in amoeba and human cells and for intrapulmonary proliferation in mice. Adapted from [53].