Figure 3.
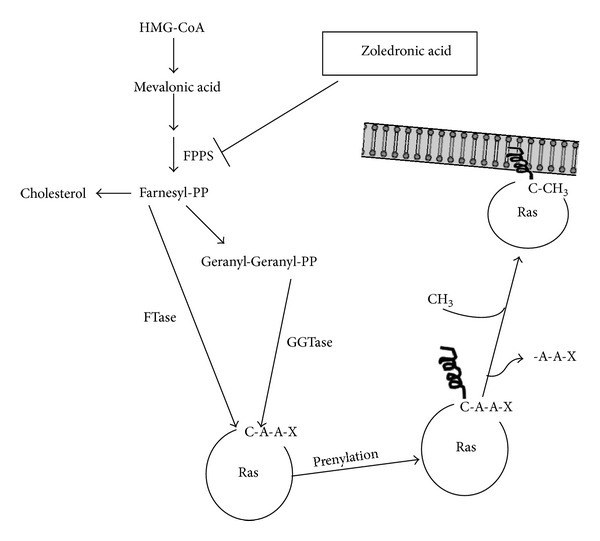
Isoprenoids are synthesized from the mevalonate pathway that starts from reaction catalyzed by the 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase (the rate-limiting reaction in cholesterol biosynthesis) which catalyzes the conversion of HMG-CoA to mevalonic acid. The pathway triggered by this reaction can lead to the synthesis of a key isoprenoid molecule, the farnesyl-pyrophosphate (Farnesyl-PP), whose formation is catalyzed by the farnesylpyrophosphate synthase (FPPS). Farnesyl-PP can be either converted by a series of reactions in cholesterol or can be transferred on target cellular proteins as Farnesyl-PP itself (reaction catalyzed by farnesyltransferase, FTase) or firstly converted in geranyl-geranyl-pyrophosphate (Geranyl-Geranyl-PP) and then transferred on cellular proteins by type I or type II geranylgeranyl-transferase (GGTase). FTase and GGTase-I catalyze the prenylation of substrates with a carboxy-terminal tetrapeptide sequence called a CAAX box, where C refers to cysteine, A refers to an aliphatic residue, and X typically refers to methionine, serine, alanine, or glutamine for FTase or to leucine for GGTase-I. Following prenylation of physiological substrates, the terminal three residues (AAX) are subsequently removed by a CAAX endoprotease, and the carboxyl group of the terminal cysteine is methyl esterified by a methyltransferase. At this moment prenyl substrates, such as Ras, are ready to be located on the inner side of the biological membranes to receive signals mediated by external factors. ZOL specifically inhibits the FPPS activity required for the synthesis of farnesyl and geranylgeranyl lipidic residues blocking prenylation of Ras that regulates the proliferation, invasive properties, and proangiogenic activity of human tumour cells.
