Figure 1.
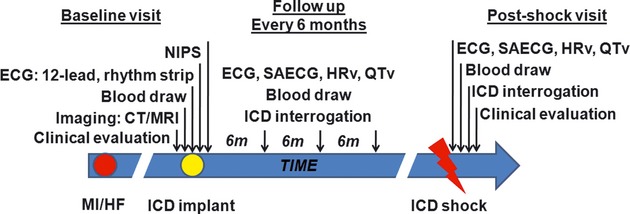
Study design of PROSE‐ICD. Baseline measurements include comprehensive history, digitized signal‐averaged ECG, 12‐lead baseline ECG, blood collection, and in some patients, cardiac computed tomography (CT) with contrast or cardiac magnetic resonance imaging (MRI) with gadolinium‐based delayed hyperenhancement. Patients are routinely evaluated either in person or via phone call every 6 months. Soon after an ICD shock, patients are encouraged to return to the clinic for further evaluation. At the visit after the shock, the device is interrogated and the event downloaded for adjudication. A 12‐lead ECG, signal‐averaged ECG, and blood collection are also collected again. PROSE‐ICD indicates Prospective Observational Study of Implantable Cardioverter‐Defibrillators; ECG, electrocardiogram; NIPS, noninvasive programmed stimulation through the ICD; MI/HF, index myocardial infarction or initial diagnosis of heart failure; SAECG, signal‐averaged ECG; HRv, heart rate variability analysis; QTv, QT interval variability analysis.
