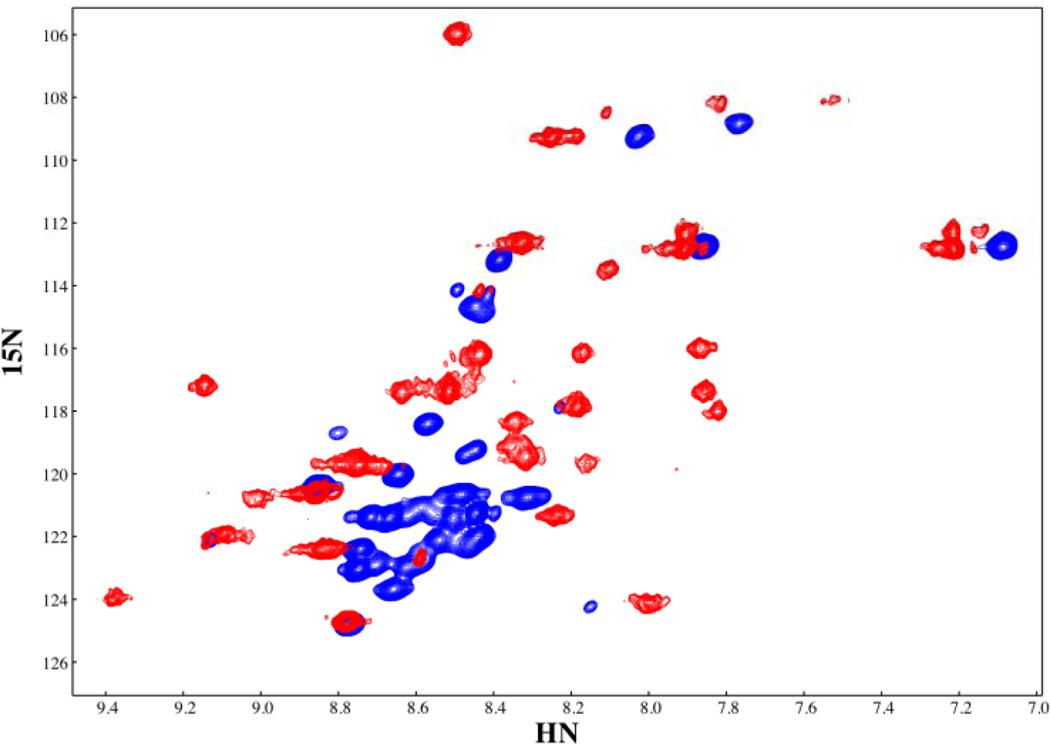
Figure 3.
The HSQC spectra of free and IκBα-bound p65(289–321). The secondary chemical shifts of bound p65(289–321) show the characteristic positive values characteristic of helical regions, conforming to the helical areas seen in these same residues in the crystal structure of the IκBα•NF-κB complex (PDB accession code 1NFI, 11). The chemical shifts for free p65(289–321) are indicative of an unfolded peptide.