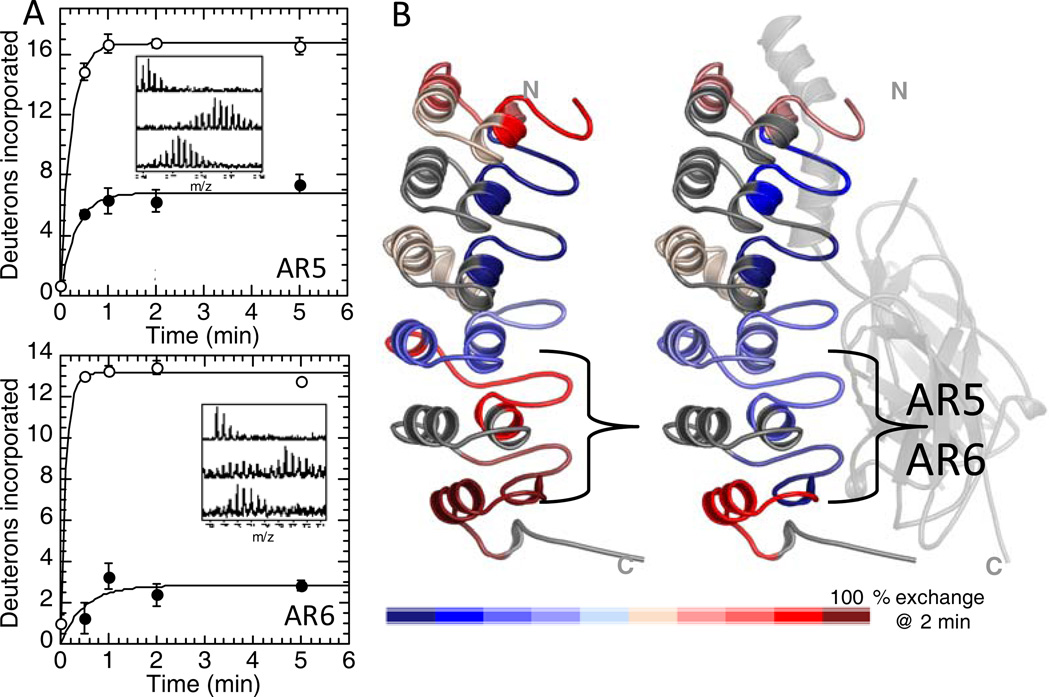
Figure 5.
(A) Native state amide H/D exchange data for the region of IκBα corresponding to the β-turn of AR5 (TOP) and AR6 (bottom) in the free-state (open circles) and in the NF-κB-bound state (closed circles). (B) Structural summary of the amide H/D exchange data (red is highly exchanging and blue is slowly exchanging) for IκBα in the free state (LEFT) and in the NF-κB-bound state (RIGHT) showing that exchange is similar for most of the IκBα molecule in each state, except for the β-turns of AR5 and AR6 that are exchanging much less in the bound state.