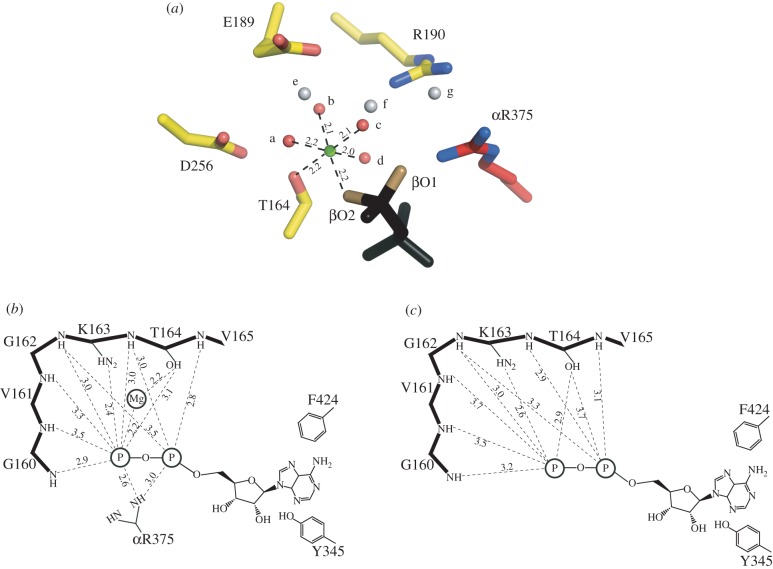
Figure 6.
Change in the coordination of a magnesium ion in the active site of yeast F1-ATPase. (a) Hexacoordination of the magnesium ion (green) in the catalytic site of the βDP-subunit. The ligands are provided by water molecule a–d (red), by the oxygen atom βO2 of ADP (or ATP), and by the β-hydroxy-group of βDP-T164. The water molecules a–d are themselves hydrogen-bonded to other water molecules e–g (grey), by the oxygen atom βO1 of the nucleotide, and by the side chain functionalities of residues βDP-E189, βDP-R190 and βDP-D256. (b) Schematic of the disposition of the magnesium ion and bound ADP in the catalytic site of the βDP-subunit. The P-loop sequence (upper left) helps to bind the magnesium ion and the nucleotide. The adenosine moiety of the nucleotide is bound in the hydrophobic pocket between the side chains of residues βDP-Y345 and βDP-F424. Distances are given in angstrom, and those to the phosphate group are to the closest oxygen atom. (c) Rotation of γ-subunit driven by hydrolysis of ATP has opened the nucleotide binding domain of the subunit, converting the βDP-site to the βE-site. The catalytic ‘arginine finger’ residue, αR375 becomes disordered, and the coordination sphere of the magnesium ion is disrupted, releasing the metal ion. In the structure of yF1-I1–53, the inhibitor protein has arrested the conversion of the site just before the formation of a fully ‘empty’ or ‘open’ site. The adenosine binding pocket is still intact, and ADP remains bound to the subunit. In the final step of the conversion of the site to a fully formed βE-site (as observed in ‘ground state’ structures of F1-ATPase), the side chain of βE-F424 rotates through 90°, releasing the ADP molecule.