Figure 7.
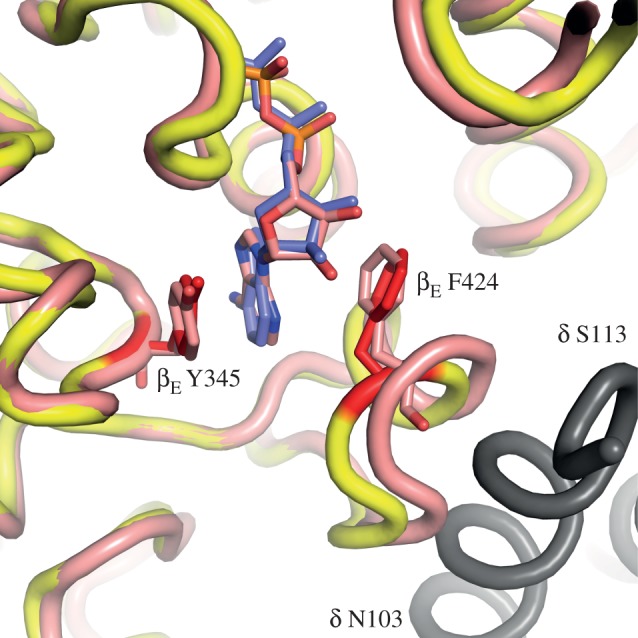
Comparison of the nucleotide binding pockets in the βE-subunits in the structures of yF1-I1–53 and bovine F1-PH. The yeast and bovine protein backbones are coloured yellow and pink, respectively. The side chains of residues βE-F424 and βE-Y345 are red in the yeast enzyme and pink in the bovine enzyme. They provide the pocket for binding the adenosine moieties of the ADP molecules (blue and pink, respectively in the yeast and bovine enzymes). In grey is shown α-helix b (residues 102–110) in the δ-subunit of an adjacent yeast F1-complex in the crystal lattice of yeast F1-I1–53. It approaches to within 4 Å of α-helix C3 carrying βE-F424 in the yeast structure. Thus, it makes a crystal contact that may influence the position of α-helix C3 in the βE-subunit of the yeast enzyme.
