FIG. 4.
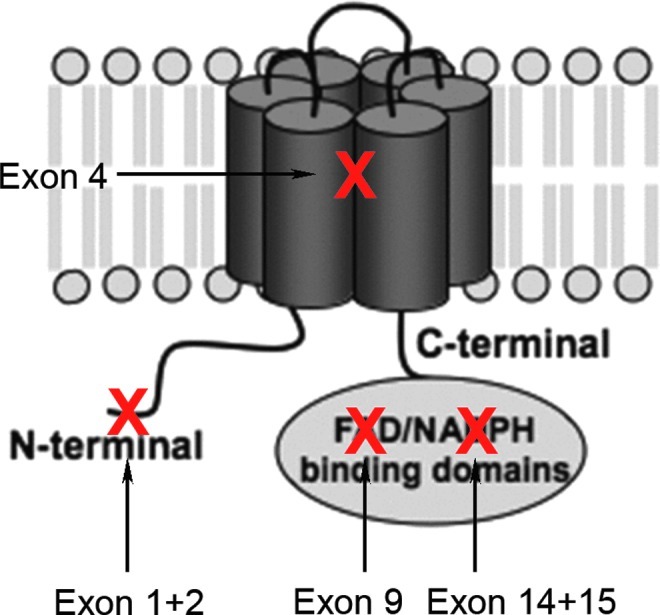
Existing NOX4 knockout (KO) models. The NOX4 protein is composed of six transmembrane domains and cytosolic binding domains for flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide phosphate (NADPH) at the C-terminus. Several groups have generated NOX4 KO models by deleting different exons of the NOX4 gene. Our group's NOX4 KO mouse was generated by deleting exons 14 and 15, which correspond to the NADPH binding domain. This likely results in the expression of a nonfunctional enzyme (33). Another NOX4 KO mouse was generated by conditionally deleting exon 9 of NOX4 in cardiomyocytes, thereby deleting the FAD binding domain (35). The splice variants of NOX4 in different cell types and tissues are not known. However, independent of which residual protein would be expressed, these two KOs would be biochemically inactive. In a third NOX4 KO model, exons 1 and 2 were deleted in an attempt to delete the NOX4 protein completely (72). Another NOX4 KO model deleted exon 4 (9). Depending on alternative splicing, these may bear the risk of truncated splice variants being expressed, which contain the NADPH/FAD reductase domain. This domain is able to generate ROS independently of the double heme domain and may, thus, in some cells not eliminate NOX4-dependent ROS formation, target it to different subcellular compartments with different or no regulation. However, this is a hypothetical risk, as the splicing in wild-type and KO mice is not fully mapped. Alternatively, truncated NOX4 proteins, that is, without NADPH and/or FAD binding sites, may affect other NOX isoforms in a dominant negative fashion, for example, by scavenging p22phox. However, there is no evidence as yet for this possibility (7).
