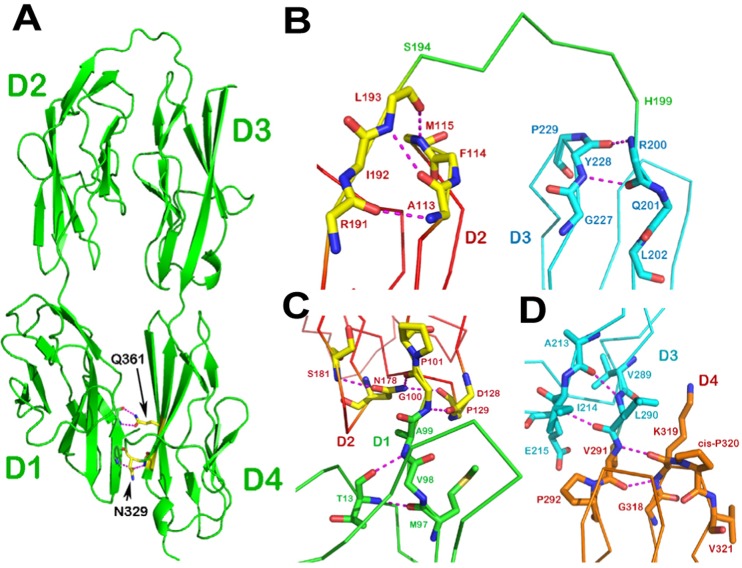
Fig. 1.
Structure of DCC horseshoe. (A) Ribbon drawing of the crystal structure of the DCC N-terminal four Ig-like domains. The molecule folds into a horseshoe configuration with a six-residue linker between domains D2 and D3. Also shown is how the conserved Asn329 and Gln361 of D4 form hydrogen bonds to the main-chain of D1 to create a specific D1/D4 interface, which defines the unique shape of the horseshoe. (B) The D2–D3 junction. At the C-terminus of D2 (in red) the last residue of D2, Leu193, participates in a pair of hydrogen bonds to Phe114 and Met115. At the N-terminus of D3 (in cyan) the first residue Arg200 is involved in a main-chain hydrogen bond with Tyr228. This clearly defines a six-residue linker (in green) from Ser194 to His199. (C) The D1–D2 junction. There is no linker present here. The last D1 residue (Ala99; in green) is still located in a part of the β sheet. The first D2 residue (Gly100; in red) is also an integrated part of D2 as it engages in a complicated hydrogen bond network. (D) The D3–D4 junction. There is no linker between these two domains either. The last D3 residue (Leu290; in cyan) is involved in a β sheet hydrogen bond network, whereas the first D4 residue (Val291; in orange) forms two main-chain hydrogen bonds with Lys319, which is on the BC loop next to the cis-Pro320. This kind of junction is commonly seen in many IgSF structures with two abutting Ig-like domains (Wang and Springer, 1998).