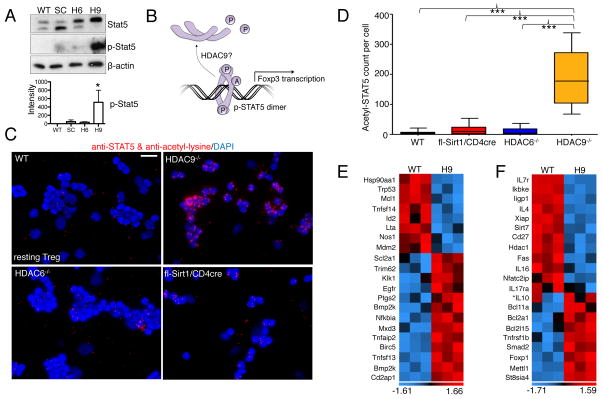
Fig. 3.
Deletion of HDAC9 stabilizes the acetylation, phosphorylation, and transcriptional activity of STAT5. (A) Analysis of Treg lysates indicated that STAT5 was relatively more phosphorylated in HDAC9−/− cells than in WT cells. Densitometry data pooled from three experiments. (B) Conceptual model of the acetylation of STAT5, which protects it from losing its transcriptionally active, phosphorylated dimeric form. (C) Proximity ligation assay showed prominent acetylation of STAT5 in HDAC9−/− Tregs compared to that in WT Tregs. (D) Quantification of the data in (C) was performed as described for Fig. 2F. (E) Microarray analysis showed STAT5-dependent signaling in HDAC9−/− Tregs compared to WT Treg (n=3/group) based on previously reported STAT5 targets (19). (F) Further STAT5 signaling as well as other transcriptional alterations promoted a suppressive phenotype in HDAC9−/− Tregs. Data are shown after z-score transformation. *IL-10 not significant. Scale bar: 10 μm. Trp, transformation-related protein; Mcl, myeloid leukemia cell differentiating protein; Tnfsf, tumor necrosis factor superfamily; Id, inhibitor of DNA; Lta, lymphotoxin-α; Nos, NO synthase; Mdm2, an E3 ubiquitin ligase; Scl2a1, GLUT1; Trim, tripartite motif; Klk, Kallikrein; ptgs, post-transcriptional gene silencing; bmp2k, bone morphogenetic protein inducible kinase-2; Cd2ap, CD2-associated protein; Ikbke, Inhibitor of nuclear factor κB kinase ε; Iigp, IFN-inducible GTPase; Xiap, X-linked inhibitor of apoptosis; Fas, CD95; Bcl, B-cell lymphoma; mettl, methyltransferase-like; St8sia, ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase-4.