Figure 1.
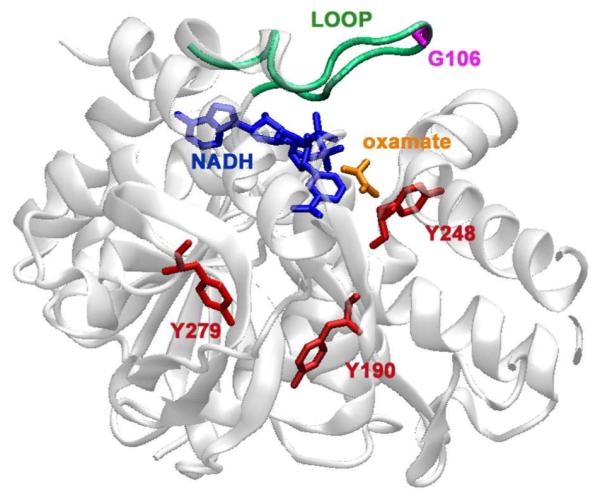
Crystal structure of a bsLDH monomer (PDB file: 1LDN) depicting the sites of mutation. In red are the sites of tyrosine to tryptophan mutations. The indole ring of the tryptophan are placed approximately 10.7 Å from the NADH ring for the Y248W mutant, 19.6 Å in Y190W and 22.3 Å in Y279W. Also highlighted are: the cofactor, NADH, in blue, oxamate in orange, and the catalytically important loop in green. In a fourth mutant, G106W, the tryptophan is placed on the loop (purple). The graphic was generated using VMD.29
