Scheme 1.
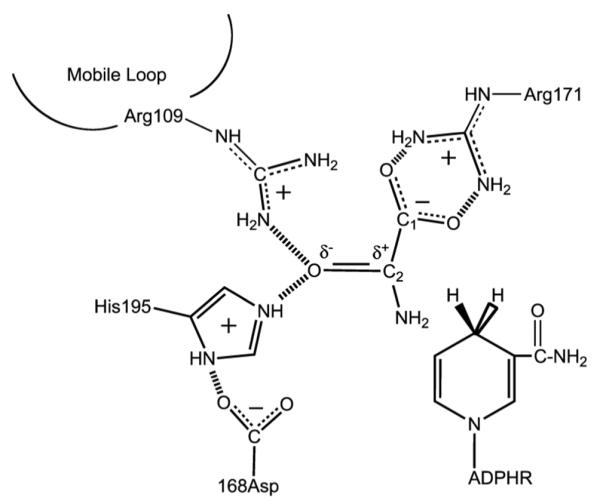
The active site contacts of pyruvate and NADH bound to LDH with key residues as determined by X-ray crystallography. The reaction catalyzed by LDH involves the direct transfer of a hydride ion from C4 of the reduced nicotinamide group of NADH to the C2 carbon of pyruvate accompanied by the protonation of pyruvate's keto-oxygen, the proton being supplied by His195. It is known that either electrostatic stabilization of the transition state in the pyruvate-lactate interconversion, which contains a highly polarized carbonyl moiety, +C-O−, or destabilization of the >C=O ground state (or a combination) is responsible for about half of the rate enhancement brought about by LDH. The other half of the rate enhancement comes about from bringing cofactor and substrate close together in a proper orientation and `activating' cofactor towards catalysis.
