Abstract
Uterine arteriovenous malformation (AVM) is a little known condition of which, to date, very few cases have been described. It has a very diverse symptomatology, even though in most cases, it is diagnosed during a severe and acute haemorrhagic event. Its treatment can vary from expectant management to hysterectomy; however, current evidence suggests that the embolisation of uterine arteries is the most effective approach, especially if fertility is to be preserved. We present a case report classified as AVM, with additional images that show the appearance of this pathology in a short span of time. This case has a number of peculiarities: unusual persistence of human chorionic gonadotropin hormone (β-HCG), asymptomatic patient, quick establishment of the lesion and its duration with unchanging characteristics and finally its spontaneous resolution without further consequences. This entity shows an aetiopathogenesis, that is, not well established or described. We discuss its physiopathology and aetiopathogenesis.
Background
It is a rare condition we have had the opportunity to study and treat with good results. We have learnt a significant lesson from our patient.
Case presentation
A healthy 31-year-old patient, nulligravida, with an unremarkable medical history, on oral contraceptive therapy for the last 10 years and normal yearly gynaecological check-ups, came to our clinic. Upon becoming pregnant, a transvaginal ultrasound in her 5 weeks of gestation displayed an intrauterine gestational sac according to gestational age, without significant findings. At week 6, a complete spontaneous miscarriage occurred, confirmed by an ultrasound scan and therefore no curettage was carried out.
Fifteen days after the miscarriage, an intermittent spotting appeared and did not stop. Through a transvaginal ultrasound scan, an irregular endometrium displayed 8 mm thickness. Ergots were prescribed and vaginal bleeding gradually slowed during the following days.
Throughout the follow-up, the patient was completely asymptomatic, and started to take oral contraceptive pills 2 months after miscarriage.
Investigations
The patient was checked again 34 days after her miscarriage. At this time, the scan showed a thickened, heterogeneous and irregular endometrium, with small echo-negative image (figure 1). After Doppler was activated, the image was seen as a hypervascularised mass. Spectral analysis showed flow velocity waves of high and low resistance and multidirectional, with aliasing phenomenon. Human chorionic gonadotropin hormone (β-HCG) levels were 125 mU/ml. Next day, heavy bleeding occurred.
Figure 1.
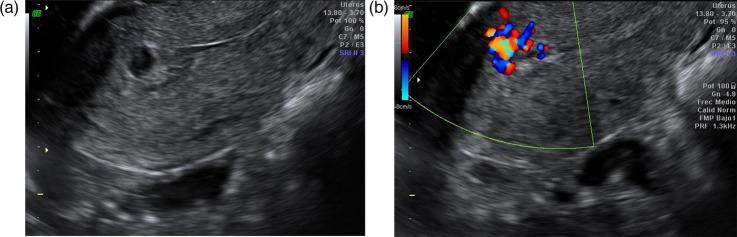
Ultrasound after 34 days of abortion. (A) A thickened, heterogeneous and irregular endometrium, with minimal echo-negative image is shown. (B) Activating Doppler, an image with multiple signs of intense colour, with turbulence in balls and aliasing is seen.
A new determination of β-HCG was requested at 44 days after miscarriage, coming back to 20 mU/ml. An ultrasound scan this time displayed a larger echo-negative image than previous examination, becoming 10 mm and corresponding to a large submucosal vessel surrounded by a highly vascularised area. This appeared like a venous vessel after analysing the waveform of flow; arterial vessels completely surrounded it with very low flow resistance.
At 56 days after miscarriage, β-HCG decreased to 4.8 mU/ml and uterine images were very similar to the previous.
Differential diagnosis
Incomplete abortion
Gestational trophoblastic disease
Treatment
We decided on an expectant management because the patient had no symptoms.
Outcome and follow-up
Two months later, she continued to be asymptomatic with conventional ultrasound images and Doppler examination similar to the one described last. In the follow-up at 8 months after miscarriage, pathological images had disappeared entirely, visualising a normal endometrial cavity, with a subendometrial area without evident vessels by Doppler.
Discussion
We consider that AVM is a clinical entity, that is, more frequent than traditionally was thought to be: much greater than the 100 cases discussed in the literature. It is an underdiagnosed entity due to the possibility of asymptomatic cases.1–10
Proof of this is that in our practice, we are surprised with uterine images shown by Doppler similar to those described in the AVM, that are seen in gynaecological screening of patients after miscarriages and deliveries, and being totally asymptomatic. Probably these images are neovessels without symptoms and self-limited in time. Could a progression of this disease exist, ranging from cases with no clinical findings to other very serious presentations that usually make up the cases reported in the literature?
Trying to understand its possible pathogenesis, we must refer to placental anatomy and physiology. We know that the primary function of the placenta is the exchange of nutrients between mother and fetus. There are two processes involved in this function, such as vasculogenesis (de novo formation of blood vessels) and angiogenesis (creation of new vessels from the pre-existing).11 However, angiogenesis is usually a rare event in adults, and this established vascular network, can only be expanded and remodelled under certain stimuli and especially in the female reproductive system: ovary, endometrium and placenta.
In the first weeks of gestation, trophoblast cells penetrate inside the decidua, reaching the myometrium, invading maternal spiral arteries. This is necessary to regulate the supply of oxygen in this period. Paradoxically, it is known that excessive vascularisation produces an increased rate of oxygen that could be detrimental to the embryo. Therefore, the presence of a continuous flow to the intervillous space is associated with early pregnancy loss.12 An enviroment of hypoxia is needed to synthesise the 1-α factor is necessary, which in turn will lead to the synthesis of other angiogenic factors and trophoblast growth by stimulating the proliferation and differentiation of endothelial progenitor cells. The vascular endothelial growth factors are the main growth factors involved in vasculogenesis and angiogenesis.13 14 Other well-studied factors involved in the processes are acidic and basic fibroblast growth factors (FGF-1 and FGF-2)15 and placental growth factor.16 They all have an important role like mitogens of endothelial cell and are expressed in different tissues, including the trophoblast.
Logically, the reason that the anomalous level of β HCG hormone continued, as it occurred in our case, is the result, in turn, of the persistence over time of trophoblastic tissue through a process of angiogenesis and rapid formation of neovessels that could remain in time after the end of that situation. We could even think of an abnormal retention of trophoblast and, consequently, vascular invasion consistent with a progression that in any case would never be considered a gestational trophoblastic disease. Indeed, in cases in which we consider indicated, hysterectomy may be performed on the workpiece study of monoclonal antibodies to β-HCG: if proved positive, would confirm the secondary source of injury.
And as we discussed earlier, excess vascularisation derived from this process is associated with increased embryonic losses and therefore the number of miscarriages. This explains why most AVM described are always being associated with a history of previous miscarriage.1 17
Controversy arises therefore, whether this entity actually has an effect on abnormal persistence of trophoblastic tissue, with different degrees of intensity and with time and therefore with a very different clinical manifestation, only those with an evident bleeding episode being diagnosed.
Learning points.
Arteriovenous malformation (AVM) is a condition that is more frequent than originally thought, since many go undiagnosed.
Pathogenesis is not well established. A number of known angiogenic and antiangiogenic factors are present but the way in which they interact and the molecular consequences of such interactions are not yet fully understood.
We recommend the application of Doppler in all examinations, especially after repeated abortions, birth trauma or previous uterine operations.
In patients with suspected AVM, it is generally not clear what examinations are necessary, what contraception method is correct, what follow-up and how often these should be performed and finally, if it is helpful to treat or not. Logically, the action to take should be individualised, case by case.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Peitsidis P, Manolakos E, Tsekoura V, et al. Uterine arteriovenous malformations induced after diagnostic curettage: a systematic review. Arch Gynecol Obstet 2011;284:1137–51 [DOI] [PubMed] [Google Scholar]
- 2.ÓBrien P, Neyastani A, Buckley AR, et al. Uterine arteriovenous malformations. from diagnosis to treatment. J Ultrasound Med 2006;25:1387–92 [DOI] [PubMed] [Google Scholar]
- 3.Machado LE, Raga F, Chagas K, et al. La malformación arteriovenosa uterina. Una lesión más frecuente y grave de lo sospechado. Prog Obstet Ginecol 2010;53:10–17 [Google Scholar]
- 4.Griffin DW, Strand EA. Arteriovenous malformation of the uterus after a midtrimester loss: a case report. J Reprod Med 2009;54:333–6 [PubMed] [Google Scholar]
- 5.Brown RL, Van Moore A, Smythe AR. Arteriographic management of uterine arteriovenous fistula. Am J Obstet Gynecol 1986;155:491–3 [DOI] [PubMed] [Google Scholar]
- 6.Barber JT, Jr, Tressler TB, Willis GS, et al. Arteriovenous malformation identification after conservative management of placenta percreta with uterine artery embolization and adjunctive therapy. Am J Obstet Gynecol 2011;204:4–8 [DOI] [PubMed] [Google Scholar]
- 7.Brown JV, III, Asrat T, Epstein HD, et al. Contemporary diagnosis and management of a uterine arteriovenous malformation. Obstet Gynecol 2008;112:467–70 [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Potti S, Jaspan D, et al. Embolization of uterine arteriovenous malformation for treatment of menorrhagia. Arch Gynecol Obstet 2009;279:229–32 [DOI] [PubMed] [Google Scholar]
- 9.Rosa E, Silva JC, De Aguiar FM, et al. Conservative management of large uterine arteriovenous malformation: case report. Fertil Steril 2008;90:2406–7 [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang RL, Nemcek AA, Jr, Skrtic Z, et al. Uterine arteriovenous malformations: primary treatment with therapeutic embolization. J Vasc Interv Radiol 1991;2:517–22 [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Bauer R, Velazquez O. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg 2005;39:293–306 [DOI] [PubMed] [Google Scholar]
- 12.Cabero L, Saldívar D, Cabrillo E. Obstetricia y Medicina Materno-Fetal. Buenos Aires, Madrid: Editorial Médica Panamericana; 2007 [Google Scholar]
- 13.Bogic L, Brace R, Cheung C. Developmental expression of vascular endothelial growth factor (VEGF) receptors and VEGF binding in ovine placenta and fetal membranes. Placenta 2001;22:265–75 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Dunk C, Ahmad S, et al. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta 2000;21(Suppl):16–24 [DOI] [PubMed] [Google Scholar]
- 15.Kingdom J, Huppertz B, Seaward G, et al. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol 2000;92:35–43 [DOI] [PubMed] [Google Scholar]
- 16.DiSalvo J, Bayne M, Conn G, et al. Purification and characterization of a naturally occurring vascular endothelial growth factor.placenta growth factor heterodimer. J Biol Chem 1995;270:7717–23 [DOI] [PubMed] [Google Scholar]
- 17.Gopal M, Goldberg J, Klein TA, et al. Embolization of a uterine arteriovenous malformation followed by a twin pregnancy. Obstet Gynecol 2003;102:696–8 [DOI] [PubMed] [Google Scholar]


