Abstract
Purpose
Tribulus terrestris has been used as an aphrodisiac. However, little is known about the effects and mechanism of action of T. terrestris on penile erection. Therefore, the effect of a T. terrestris extract and the mechanism of action of the extract on relaxation of the corpus cavernosum (CC) were investigated. The erectogenic effects of an oral preparation of the extract were also assessed.
Materials and Methods
The relaxation effects and mechanism of action of the T. terrestris extract on rabbit CC were investigated in an organ bath. The intracavernous pressure (ICP) was calculated after oral administration of the extract for 1 month to evaluate whether the relaxation response of the CC shown in the organ bath occurred in vivo. Additionally, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) were measured in the CC by immunoassay. Smooth muscle relaxation was expressed as the percentage decrease in precontraction induced by phenylephrine. The ICP was also assessed in rats after oral administration of the extract for 1 month, and changes in concentrations of cGMP and cAMP were monitored.
Results
Concentration-dependent relaxation effects of the extract on the CC were detected in the organ bath study. Relaxation of the CC by the T. terrestris extract was inhibited in both an endothelium-removed group and an L-arginen methyl ester pretreatment group. The ICP measured after oral administration of the T. terrestris extract for 1 month was higher than that measured in the control group, and a significant increase in cAMP was observed in the T. terrestris extract group.
Conclusions
The T. terrestris extract induced concentration-dependent relaxation of the CC in an organ bath. The mechanism included a reaction involving the nitric oxide/nitric oxide synthase pathway and endothelium of the CC. Moreover, in an in vivo study, the T. terrestris extract showed a significant concentration-dependent increase in ICP. Accordingly, the T. terrestris extract may improve erectile function.
Keywords: Penile erection, Smooth muscle, Tribulus
INTRODUCTION
Recently developed phosphodiesterase type-5 (PDE-5) inhibitors have been widely used as first-line therapeutics to treat erectile dysfunction (ED). Although large, multicenter clinical trials have shown the efficacy and tolerability of these drugs in patients with ED of various etiologies with a broad range of severity, 30% to 35% of patients fail to respond. The use of PDE-5 inhibitors may result in side effects, including visual disturbances, headache, facial flushing, rhinitis, and indigestion. Other treatments for ED include penile injection therapy or penile implants. However, such methods are invasive and irreversible and are not widely used [1]. Thus, there is a continuing need for the development of new noninvasive and effective therapies to treat patients with ED.
Despite the remarkable developments of modern medicine, many people are still favorably disposed toward herbal medicines owing to the aggressive treatment protocols, toxicity, and drug tolerance associated with modern therapies. The widespread use of herbal medicines, however, requires scientific verification of their indications and effects by use of modern medical analysis.
Tribulus terrestris is a perennial creeping herb that is broadly distributed in Mediterranean, subtropical, and desert climates worldwide. It has been used since ancient times in traditional folk medicine as an aphrodisiac and to treat urinary tract infections, inflammation, and other ailments [2]. Animal studies have found that T. terrestris is helpful as an aphrodisiac [3]. The treatment of castrated rats with a Tribulus extract was previously shown to increase the weight of the prostate and intracavernous pressure (ICP). Improved sexual behavior was also detected, as evidenced by an increase in mounting frequency. However, much controversy surrounds the possible mechanisms of action and the therapeutic applications of T. terrestris extracts [4]. Furthermore, little is known about the effects and mechanism of action of T. terrestris on penile erection.
In this study, we investigated the effect of a T. terrestris extract on relaxation of the corpus cavernosum (CC) in tissue from rabbits and in rats. The mechanism of action of the extract was also assessed. We further examined the erectogenic effects of the extract after oral administration.
MATERIALS AND METHODS
1. T. terrestris extraction
T. terrestris was purchased at a local market in Korea. An amount of 100 g of the T. terrestris fruit was ground and extracted with 1 L of 90% ethanol at 80℃ for 2 hours and then filtered through Whatman No. 2 filter paper. The filtrates were evaporated in a rotary evaporator (Eyela-NE, Rikakikai Co., Tokyo, Japan) under reduced pressure. The yield of T. terrestris fruit extract was 25% (wt/wt). The aqueous extracts were filtered and lyophilized (Thermo Fisher Scientific Inc., Rockford, IL, USA) to yield 55% (wt/wt) of the crude extract. These extracts were preserved in a refrigerator and used in the studies described below.
2. Organ bath CC study
New Zealand white male rabbits weighing 2.5 to 3.0 kg were used (n=8/group). All the animals were cared for in accordance with the National Research Council publication Guide for the Care and Use of Laboratory Animals. The animal experiments were approved by the Institutional Animal Care and Use Committee of the research institute at the university. The rabbits were sacrificed with an overdose of ketamine hydrochloride (50 mg/kg) injected into the marginal vein of the ear, the penis was dissected immediately, and sections of the CC (2 mm×2 mm×10 mm) were prepared. The sections were then transferred to an organ bath; one end was connected to a muscle fixation ring and the other end was connected to an isometric tension transducer (SG-10). The sections were connected to a force displacement transducer (TSD 125C, Biopac Inc., Goleta, CA, USA), which is an isometric tension transformer, and the signals were recorded on a personal computer by using a four-channel data acquisition and analysis system for Windows (MP36R 4-Channel Systems, Biopac Inc.). The signals were relayed to a physiography instrument (Power-Lab, ADI Instruments, Sydney, Australia) and measured. Chart 5 software (ADI Instruments) was used for real-time monitoring of tension. Krebs-Henseleit (KH) solution was used for the organ bath at 37℃ and pH 7.4 and gassed continuously with 95% O2/5% CO2. With the initial tension of each section maintained at approximately 2 g, the KH solution was changed approximately every 30 minutes (total equilibration period, 2 hours) and allowed to reach a stable condition. Once a stable condition was reached, phenylephrine (PE) was added and the contraction level was observed. Each section was progressively stretched to the optimal point on its length-tension curve as determined by the active tension developed in response to PE. A stable condition was restored by washing the preparation with KH solution three times. These steps were repeated, and contracture within 100%±10% of the previous contraction was defined as the ideal resting optimal isometric tension. While maintaining resting optimal isometric tension, the tissues were contracted by PE (5×10-6 M) pretreatment within the bath. A T. terrestris extract in five-fold dilutions (0.25 to 4 mg/mL) was added, and the relaxation level was assessed by observing the change in the tension curve.
The relaxation reactions were investigated in endothelium-intact or -denuded tissue groups to examine the mechanism by which the T. terrestris extract induced CC relaxation. Endothelium-intact or -denuded tissues from the CC were incubated for 30 minutes in KH solution containing various drugs. Next, the tissues were contracted with PE (5×10-6 M), during which plateau state contraction was attained. The relaxation reaction was then observed following the addition of increasing concentrations of T. terrestris extract. The drug probes included N(G)-nitro-L-arginine methyl ester (L-NAME; 10-5 M, nitric oxide [NO] synthesis inhibitor), propronolol (10-5 M, β-receptor blocker), indomethacin (3×10-5 M, cyclooxygenase inhibitor), glibenclamide (10-5 M, K+ATP channel inhibitor), 4-aminopyridine (10-5 M, membrane potential-dependent K+ channel inhibitor), and methylene blue (10-5 M, guanyl cyclase inhibitor) [5-7]. Eight sections were used for each group. The CC sections were pretreated with 3 mL of 0.3% 3-[(3-cholamide propyl)]-1-propane sulfonate (CHAPS) for 20 seconds to remove all vascular endothelial cells. The sections were rubbed lightly by using the thumb and index finger for 20 seconds and washed with KH solution, and the cells were removed by gently rolling the sections on dry paper. Removal of the vascular endothelial cells was confirmed by the failure of the loss of tissue contraction induced by acetylcholine (10-5 M) in response to PE (5×10-6 M).
3. Measurement of the ICP after long-term oral administration
The CC relaxation response shown in the organ bath was studied to determine whether it was expressed in vivo. Forty Sprague-Dawley male rats weighing 280 to 320 g were allocated to six groups according to the oral dosage received: control, 2.5, 5, 10, 50, and 100 mg/kg (n=8 each). Next, the groups were orally administered the same T. terrestris extract every day for 1 month before measurement of the ICP.
The rats were anesthetized with ketamine (50 mg/kg) injected intraperitoneally. The animals were placed in the supine position on an operating table, a catheter (polyethylene-50 tube) was inserted into the carotid artery, and blood pressure (BP) was monitored. The prostate was exposed by a midline incision of the abdomen. Additionally, the pelvic ganglion, located on the posterolateral side of the prostate, was assessed, and the pertinent pelvic nerves and cavernosal nerves were assessed and dissected without injury. The cavernosal nerves were stimulated by using an electric stimulator and platinum electrodes. To assess an erection in response to electrical stimulation of the nerves, the penis was dissected up to the penile crura. The tunica albuginea of the penis was assessed by dissecting the ischial corpus cavernosal muscles surrounding the penile crura, and a 25-gauge needle was installed (polyethylene-50 tube pretreated with 250 U/mL heparin) in the CC [8].
BP and ICP were measured by using a BP manometer transducer and recorder (PowerLab, ADI Instruments). Chart 5 software (ADI Instruments) was used for real-time BP monitoring. Electrical stimulation was performed at a voltage of 5 V with a 60-second duration.
Pressure was measured before, during, and after stimulation to examine the hemodynamic reaction in response to autonomic nerve stimulation. Additionally, to examine the reactions induced by nerve stimulation, the percentage of maximum ICP/mean systemic arterial blood pressure (max ICP/MAP×100) was measured by stimulating the CC nerves, and differences among the groups were compared.
4. Measurement of cGMP and cAMP in rabbit CC
Contraction of the smooth muscle sections was equalized for 1 hour in KH solution and then induced with PE (5×10-6 M), after which the tissues were exposed to the T. terrestris extract for 10 minutes. The tissues were frozen rapidly in liquid nitrogen and homogenized in ice-cold 6% trichloroacetic acid. The homogenized tissues were centrifuged for 15 minutes, and cellular proteins in the supernatant were separated by using conventional methods. The samples were divided into a normal CC group and experimental groups (0.25, 0.5, 1, 2, and 4 mg/mL T. terrestris extract), and the concentrations of released cGMP (n=8/group) and cAMP (n=8/group) were measured by immunoassay. The immunoassay was based on the competitive binding technique, in which the cGMP and cAMP in a sample compete with a fixed amount of horseradish peroxidase-labeled cGMP and cAMP for sites on rabbit polyclonal antibodies. During incubation, the polyclonal antibodies become bound to a microplate coated with goat antirabbit antibodies. Following a wash to remove excess conjugate and unbound sample, a substrate solution was added to determine the bound enzyme activity. Color development was stopped, and the absorbance was read at 450 nm. The color intensity was inversely proportional to the concentrations of cGMP and cAMP in the sample.
5. Drugs and solutions
PE, L-NAME, glibenclamide, 4-aminopyridine, indomethacin, methylene blue, propranolol, and CHAPS were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The composition of the KH solution (pH 7.4) was (mM/L) as follows: NaCl, 118.1; NaHCO3, 25; KCl, 4.6; KH2PO4, 1.2; CaCl2, 2.5; MgSO4, 1.21; and glucose, 11.0.
6. Data analysis
The statistical significance was analyzed by a t-test and one-way analysis of variance followed by Scheffe's F test by using the SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as the mean±standard deviation. A p-value <0.05 indicated statistical significance.
RESULTS
1. CC relaxation response and the mechanism of action
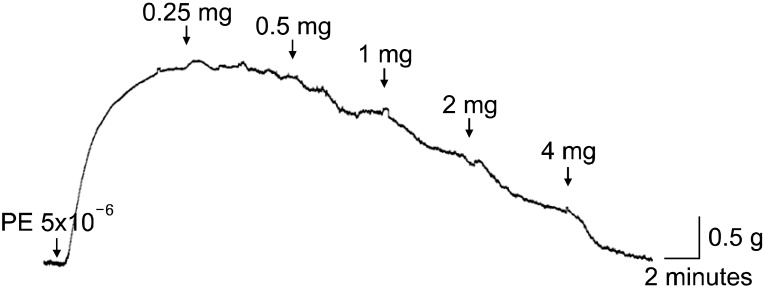
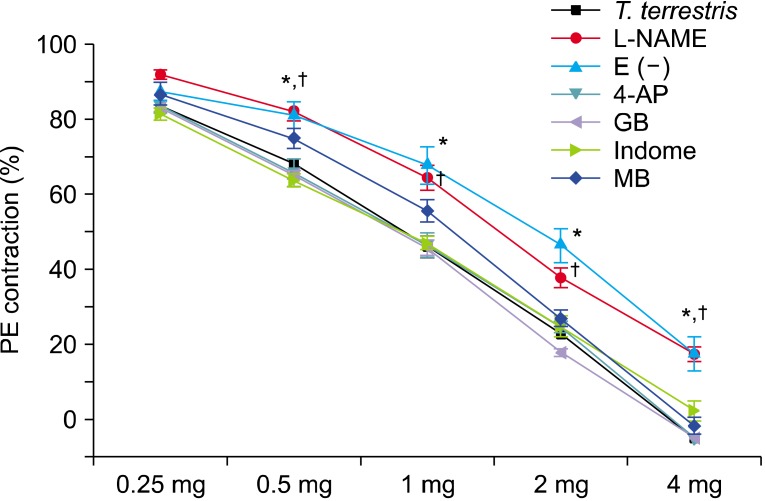
The T. terrestris extract produced a concentration-dependent relaxation response at 0.25 to 4 mg/mL (Fig. 1). Glibenclamide (10-5 M, KATP blocker), 4-aminopyridine (10-5 M, K+ channel blocker), methylene blue (10-5 M, guanylate cyclase inhibitor), indomethacin (3×10-5 M, prostaglandin pathway cyclooxygenase inhibitor), and L-NAME (10-5 M, NO/NO synthase [NOS] inhibitor) were each administered respectively before extract treatment to investigate the mechanism of action of the relaxation response. The group of endothelium-denuded tissues from the CC was also investigated. Relaxation of the CC was inhibited in the endothelium-removed and L-NAME pretreatment groups (p<0.05) (Fig. 2).
FIG. 1.
Relaxation effects of the Tribulus terrestris extract (mg/mL) on phenylephrine (PE) contraction (5×10-6 M) in rabbit corpus cavernosum. The T. terrestris extract showed a concentration-dependent relaxation response beginning at 0.25 mg/mL (p<0.05).
FIG. 2.
Effects of a Tribulus terrestris extract on the relaxation response of the rat corpus cavernosum (CC). The relaxation response of the CC was suppressed only by the nitric oxide synthesis inhibitor L-arginine methyl ester (L-NAME) and endothelium-denuded [E (-)] CC. Pretreatment with indomethacin (Indome), glibenclamide (GB), 4-aminopyridine (4-AP), or methylene blue (MB) for 30 minutes did not suppress the relaxation of the CC by the T. terrestris extract (p<0.05, n=8/group). PE, phenylephrine. *,†p<0.05.
2. Changes in the ICP after oral administration of the T. terrestris extract for 1 month
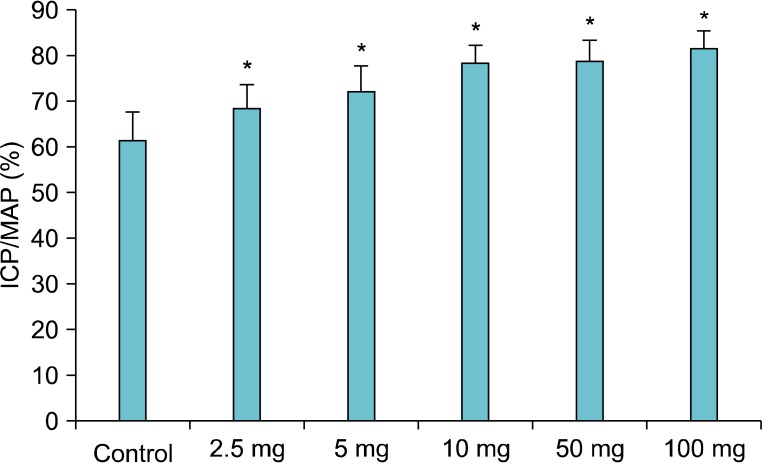
The T. terrestris extract was orally administered every day at 2.5, 5, 10, 50, or 100 mg/kg for 1 month. Next, the ICP/MAP (%) was measured during neural stimulation of the CC. After 1 month of oral administration, a significant concentration-dependent increase in ICP/MAP was observed in accordance with the increasing dosage of the T. terrestris extract (p<0.05). The increase in ICP was observed at a dose of 2.5 mg/kg·d T. terrestris extract, and the maximum effect occurred at 100 mg/kg·d compared with that in the control group (p<0.05) (Fig. 3).
FIG. 3.
Changes in intracavernous pressure (ICP) after oral administration of the Tribulus terrestris extract for 1 month. The graph shows an increase in ICP in response to carvernosal nerve stimulation in all groups. The maximum ICP/mean systemic arterial blood pressure (MAP) showed a significant concentration-dependent increase beginning at 2.5 mg/kg compared with that in the control group (p<0.05, n=8/group). *p<0.05.
3. Changes in cAMP and cGMP in the CC
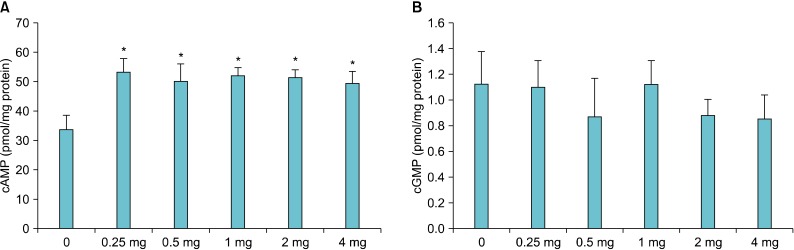
The cAMP concentration in the CC increased significantly after administration of the T. terrestris extract. However, the maximum concentration was observed at 0.5 mg; at 1, 2, and 4 mg, the values were lower than those at 0.5 mg. The cAMP concentrations at 0.5 and 4 mg were lower than those at 1 and 2 mg, revealing no concentration-dependent pattern (Fig. 4A). The cGMP concentration in the CC after administration of the T. terrestris extract was not statistically significant (Fig. 4B).
FIG. 4.
Changes in the cyclic adenosine monophosphate (cAMP) (A) and cyclic guanosine monophosphate (cGMP) (B) concentrations in the corpus cavernosum (CC) of rats (n=8/group) after oral administration of the Tribulus terrestris extract for 1 month. The cAMP concentration showed a statistically significant increase compared with that in the control group, but the difference was not significant for cGMP. *p<0.05.
DISCUSSION
T. terrestris is a flowering plant in the family Zygophyllaceae. It is native to warm temperate and tropical regions of the Old World in Southern Europe, Southern Asia, Africa, and Australia. T. terrestris, also called "puncture vine," is used worldwide to improve sexual function in humans. In Turkey, it is commonly used in folk medicine to treat abnormal BP and cholesterol levels. In Europe, it has been used in folk medicine throughout history, as far back as the Greeks, for wide-ranging conditions such as headache, nervous disorders, and sexual dysfunction. The herb has also been touted for use as a liver, kidney, urinary, and cardiovascular remedy in China and India.
T. terrestris has been used as an aphrodisiac and enhancer of sperm production as well as an alternative to hormone replacement therapy in aging men and women [2]. However, much debate surrounds the possible mechanisms of action and the therapeutic applications of T. terrestris extracts. Results published by Gauthaman et al. [3,9] indicate that T. terrestris improves some aspects of male sexual behavior and enhances spermatogenesis in rats. Additionally, clinical data indicate the stimulatory effects of T. terrestris on sperm quantity and quality and improved sexual response in men [10]. Increased androgen levels have also been reported following T. terrestris administration in nonhuman primates, rats, and rabbits [2,11], but most of these effects were short-lived and showed no clear dose-response relationship. In addition, there is no consensus on the mechanism underlying the effects of T. terrestris on sexual performance and spermatogenesis. Recently, Martino-Andrade et al. [4] demonstrated that T. terrestris has no intrinsic hormonal activity, because it was unable to stimulate endocrine-sensitive organs in either male or female rats. They also demonstrated that the administration of T. terrestris to intact male rats for 28 days did not change serum testosterone levels and did not produce quantitative changes in the fecal excretion of androgenic metabolites.
Because T. terrestris has been used to improve sexual function in various regions of the world, we presumed that it might have a direct relaxation effect on the CC. The results of our study showed that the T. terrestris extract caused relaxation of the cavernous smooth muscle in a concentration-dependent manner, and that the mechanism included a reaction involving the NO/NOS pathway in the CC endothelium. The traditional understanding of the action of NO in the penis is that NO is constitutively produced and released from autonomic nerve terminals and endothelial cells in corporal tissue. It diffuses locally into adjacent smooth muscle cells and binds with intracellular guanylate cyclase, which serves as a physiological "receptor" [12]. This binding induces a conformational change in guanylate cyclase, activating the enzyme, so that it catalyzes the conversion of guanosine triphosphate to cGMP. cGMP then operates through a cGMP-dependent protein kinase to regulate the contractile state of the corporal smooth muscle [13].
Although the mechanism of action of the T. terrestris extract occurred through NO/NOS signaling in the CC in this study, a significant increase in cGMP within the tissues was not confirmed. The cAMP concentration was significantly increased in the T. terrestris extract-treated group compared with that in the control group.
However, the cAMP concentration did not reveal a concentration-dependent relaxation effect in accordance with the concentration of the T. terrestris extract. Because the T. terrestris extract was composed of many chemical compounds rather than a single chemical compound, other CC relaxation signal transduction pathways not confirmed in this study might be necessary for the effect. Therefore, additional experimental verification is needed to identify how this phenomenon occurs.
A significant concentration-dependent increase in ICP was observed in accordance with the increasing dosage of the T. terrestris extract, similar to our organ bath experimental results. The oral administration of a T. terrestris extract in clinical practice is expected to improve erectile function. Although further clinical research is needed, the extract shows promise as an erectogenic agent.
CONCLUSIONS
The T. terrestris extract showed a concentration-dependent relaxation effect on the CC in an organ bath. The mechanism involved NO/NOS signaling in the CC endothelium. Moreover, an in vivo study after 1 month of oral administration of the T. terrestris extract showed a significant concentration-dependent increase in ICP compared with that in the control group. Accordingly, the T. terrestris extract may improve erectile function.
Footnotes
The authors have nothing to disclose.
References
- 1.Gholamine B, Shafiei M, Motevallian M, Mahmoudian M. Effects of pioglitazone on erectile dysfunction in sildenafil poor-responders: a randomized, controlled study. J Pharm Pharm Sci. 2008;11:22–31. doi: 10.18433/j3tg6h. [DOI] [PubMed] [Google Scholar]
- 2.Gauthaman K, Ganesan AP. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction: an evaluation using primates, rabbit and rat. Phytomedicine. 2008;15:44–54. doi: 10.1016/j.phymed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Gauthaman K, Adaikan PG, Prasad RN. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002;71:1385–1396. doi: 10.1016/s0024-3205(02)01858-1. [DOI] [PubMed] [Google Scholar]
- 4.Martino-Andrade AJ, Morais RN, Spercoski KM, Rossi SC, Vechi MF, Golin M, et al. Effects of Tribulus terrestris on endocrine sensitive organs in male and female Wistar rats. J Ethnopharmacol. 2010;127:165–170. doi: 10.1016/j.jep.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Bagcivan I, Gokce G, Yildirim S, Sarioglu Y. Investigation of the mechanism of nicotine induced relaxation in rabbit corpus cavernosum in vitro. Urol Res. 2004;32:209–212. doi: 10.1007/s00240-004-0404-z. [DOI] [PubMed] [Google Scholar]
- 6.Utkan NZ, Utkan T, Sarioglu Y, Canturk NZ, Okay E. Investigation of the mechanism of nicotine-induced relaxation in guinea pig gallbladder. J Surg Res. 2003;110:272–275. doi: 10.1016/s0022-4804(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 7.Skiker M, Mekhfi H, Aziz M, Haloui B, Lahlou S, Legssyer A, et al. Artemisia herba-alba Asso relaxes the rat aorta through activation of NO/cGMP pathway and K(ATP) channels. J Smooth Muscle Res. 2010;46:165–174. doi: 10.1540/jsmr.46.165. [DOI] [PubMed] [Google Scholar]
- 8.Diederichs W, Stief CG, Lue TF, Tanagho EA. Norepinephrine involvement in penile detumescence. J Urol. 1990;143:1264–1266. doi: 10.1016/s0022-5347(17)40251-5. [DOI] [PubMed] [Google Scholar]
- 9.Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncturevine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complement Med. 2003;9:257–265. doi: 10.1089/10755530360623374. [DOI] [PubMed] [Google Scholar]
- 10.Arsyad KM. Effect of protodioscin on the quantity and quality of sperms from males with moderate idiopathic oligozoospermia. Medika. 1996;22:614–618. [Google Scholar]
- 11.El-Tantawy WH, Temraz A, El-Gindi OD. Free serum testosterone level in male rats treated with Tribulus alatus extracts. Int Braz J Urol. 2007;33:554–558. doi: 10.1590/s1677-55382007000400015. [DOI] [PubMed] [Google Scholar]
- 12.Burnett AL. Nitric oxide in the penis: physiology and pathology. J Urol. 1997;157:320–324. [PubMed] [Google Scholar]
- 13.Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]