Table 3.
Comparison of the experimental 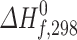 (exp.) and theoretical
(exp.) and theoretical 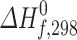 (calc.) values of the enthalpy of formation of the reactants CH3X and products CH2X, (X = F, Cl and Br) obtained at the G2 level
(calc.) values of the enthalpy of formation of the reactants CH3X and products CH2X, (X = F, Cl and Br) obtained at the G2 level
| Molecular |
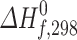 (calc.) (calc.) |
 (exp.) a) (exp.) a)
|
|---|---|---|
| system | (kJ mol−1) | (kJ mol−1) |
| CH3F | −237.7 | −238 ± 8 |
| CH3Cl | −81.4 | −81.9 ± 0.6 |
| CH3Br | −32.0 | −37.7 ± 1.5 |
| CH2F | −28.1 | −32 ± 8 |
| CH2Cl | 120.4 | 117.3 ± 3.1 |
| CH2Br | 174.9 | 169 ± 4 |
a)from ref. 12
