Figure 4. Cocrystal Structure of gp67/σA4.
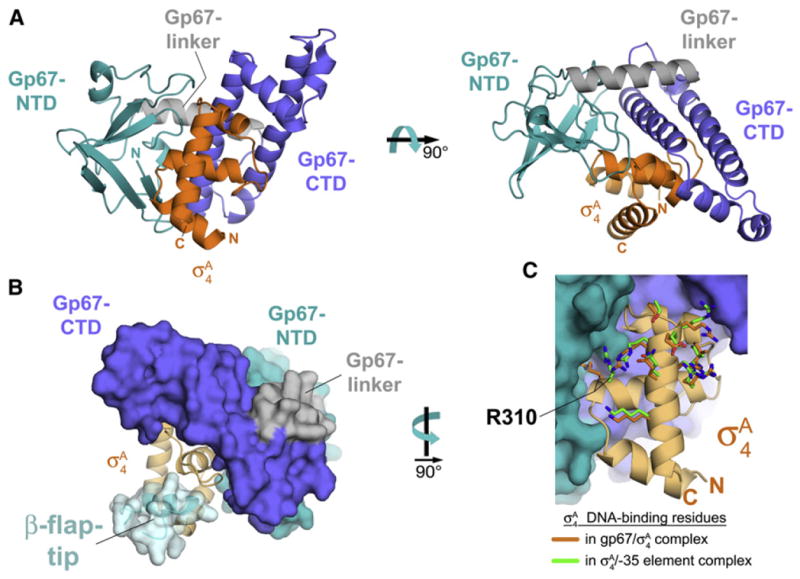
(A) Cocrystal structure of gp67 bound to σA4 (orange). Two orthogonal views are shown. Proteins are shown in ribbon format.
(B) Structural modeling indicates that the RNAP β-flap-tip/σA4 interaction is compatible with the presence of gp67. Gp67 is shown as a molecular surface, color-coded as in (A). The σA4 is shown as a light orange ribbon. The RNAP β-flap-tip, modeled by superimposing σA4 from the Thermus aquaticus RNAP holoenzyme (Murakami et al., 2002b), is shown as a light blue ribbon with a transparent molecular surface (cyan).
(C) Gp67 does not contact most residues of σA4 that interact with –35-element DNA. Shown is a close-up view of Sau σA4 (light orange ribbon) in the complex with gp67 (molecular surface, color-coded as in [A]). Side chains of residues that directly interact with –35-element DNA are shown from the gp67/Sau σA4 structure (orange) and from the superimposed Taq σA4/-35 element DNA complex (green side chains; PDB ID code 1KU7; Campbell et al., 2002). Only the side chain of Sau σA4 R310 (corresponding to Taq σA 4 R379/Eco σ70 R554) is altered through an interaction with gp67.
See also Figure S4 and Tables S1 and S2.
(C) Gp67 does not inhibit transcription from selected DNA replication promoters in vitro. The indicated bands were quantified by phosphorimagery; relative activity (versus holoenzyme alone) is denoted below each lane.
(D) Selected genes inhibited by gp67 in vivo. RNA-seq data were visualized as above from the csp1, csp2, and pstp loci.
(E) Gp67 inhibits transcription in vitro from the selected promoters that are susceptible in vivo when wild-type (WT) σA is used, but not the quadruple mutant σA that does not interact with gp67 (M). The indicated bands were quantified by phosphorimagery; relative activity (versus holoenzyme alone) is denoted below each lane.
See also Figures S2 and S3, and Table S3.
