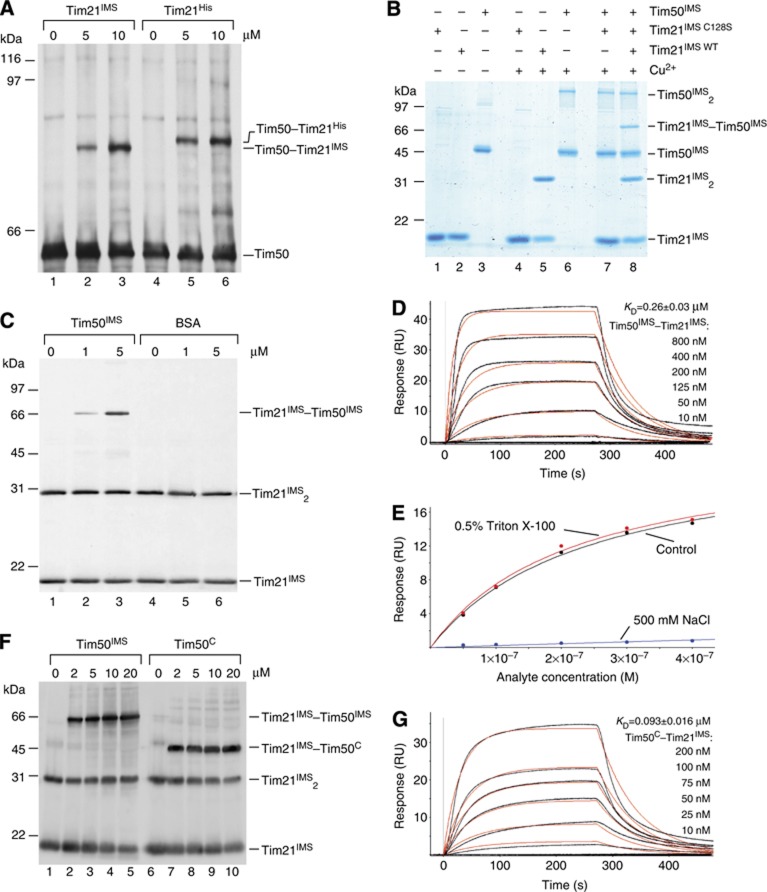
Figure 2.
Tim21 interacts directly with Tim50 in organello and in vitro. (A) Wild-type mitochondria were osmotically swollen and subjected to CuSO4 crosslinking in the presence of indicated concentrations of purified Tim21IMS or its His-tagged form Tim21His. Crosslinking-adducts were detected by immunoblotting against Tim50. (B) Purified recombinant proteins (25 μM Tim21IMS or Tim21C128S and 10 μM Tim50IMS) were crosslinked in the presence of 5 mM CuSO4. Crosslinking-adducts were visualized by colloidal Coomassie staining. (C) Indicated amounts of purified Tim50IMS or bovine serum albumin (BSA) were mixed with recombinant Tim21IMS (final concentration 1 μM) and crosslinked by adding of CuSO4, followed by immunoblotting and decoration with anti-Tim21 antibody. (D) Tim50IMS-His was immobilized on a chip and the SPR response was recorded after adding indicated concentrations of Tim21IMS. A typical sensogram is shown. Black lines, observed binding; red, calculated ka/kd fitting of kinetic data. KD is presented as mean±s.e.m. (n=3 for each measurement). (E) The SPR response was recorded as in (D) in three different buffers: control (50 mM HEPES, pH 7.4, 150 mM NaCl, and 50 μM EDTA); 0.5% Triton X-100 (50 mM HEPES, pH 7.4, 150 mM NaCl, 50 μM EDTA, and 0.5% Triton X-100); 500 mM NaCl (50 mM HEPES, pH 7.4, 500 mM NaCl, and 50 μM EDTA). Titration isotherms of maximal response versus analyte concentration are shown (data fitting is based on a model of a simple bimolecular interaction). (F) In all, 1 μM Tim21IMS was mixed with indicated amounts of Tim50IMS or Tim50C and crosslinked by CuSO4. Immunoblotting with anti-Tim21 antibody was used to detect crosslinking-adducts. (G) Interaction between Tim50C-His immobilized on a Ni2+-chelator chip and Tim21IMS was analysed by SPR, as in (D).