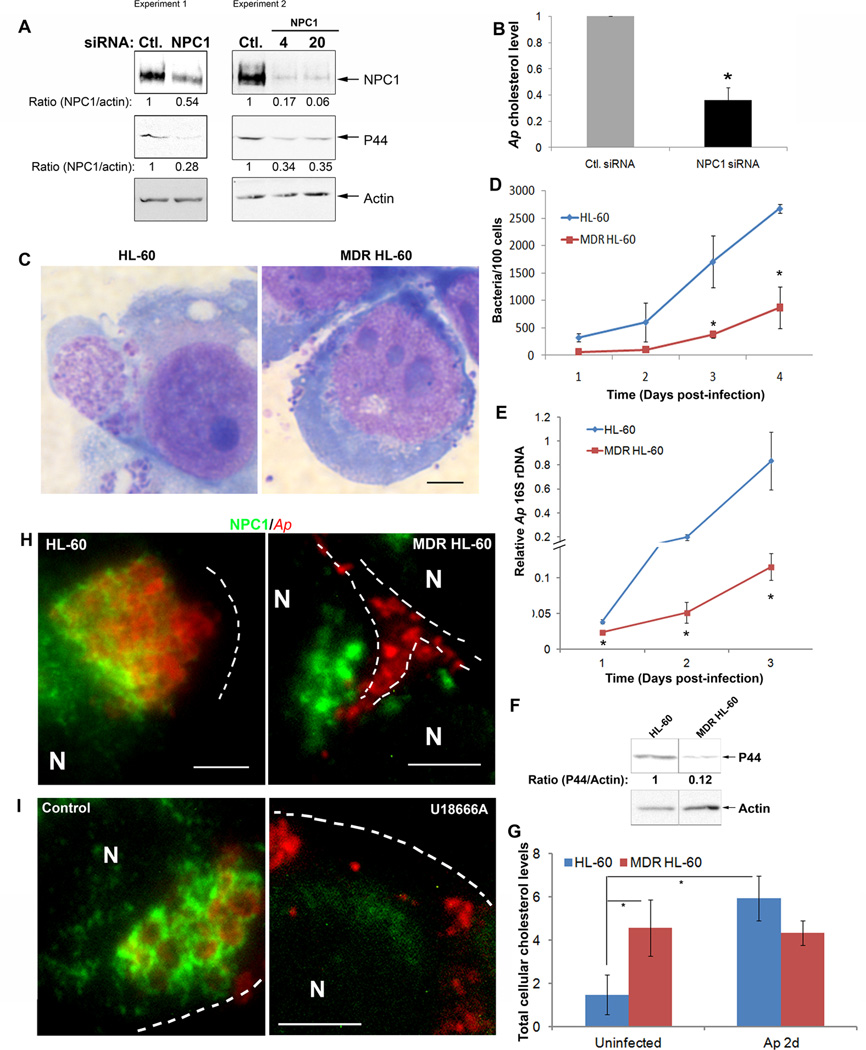
Fig. 5. NPC1 is essential for A. phagocytophilum infection.
A. RF/6A cells were transfected with a control (Ctl.) siRNA or a siRNA specific for NPC1. One day after transfection, A. phagocytophilum was added to the cells and incubated for an additional 2 days. The cell samples were lysed and subjected to western blotting using antibodies against NPC1 and A. phagocytophilum P44. Actin was used as the protein input control to normalize each sample. The amounts of NPC1 and P44 (relative to actin) were determined by densitometry. The values under the bands show the relative ratios of band intensities, with the ratios of those from samples transfected with control siRNA arbitrarily set as 1. Results are representative of four independent experiments. Ap, A. phagocytophilum.
B. RF/6A cells were transfected with siRNA followed by infection with A. phagocytophilum. At day 2 pi, host cell-free bacteria were purified, and bacterial cholesterol was measured. Each cholesterol amount was normalized to the amount of bacterial 16S rDNA as determined by quantitative PCR, and bacterial cholesterol level from control (Ctl.) siRNA-treated cells was arbitrarily set as 1. Results are representative of three independent experiments. Ap, A. phagocytophilum.
C–F. A. phagocytophilum-infected multidrug resistant (MDR) and drug-sensitive HL-60 cells were harvested at the indicated time and observed by light microscopy (C), and bacterial load was determined by counting the number of bacteria in the cells stained by Diff-Quik (D), by measuring bacterial 16S rDNA by quantitative real-time PCR (E), and by western blotting for the outer membrane protein P44 using antibody 5C11 (F). Actin was used as the protein input control to normalize each sample. Data are expressed as mean ± standard deviation (n = 3) and are representative of three independent experiments with similar results. *, P < 0.05; **, P < 0.01 (unpaired two-tailed t-test).
G. Total cellular cholesterol levels in MDR and drug-sensitive HL-60 cells with and without A. phagocytophilum infection were measured and normalized to the total cellular protein levels. Data are expressed as mean ± standard deviation (n = 3) and are representative of three independent experiments with similar results. *, P < 0.05 (unpaired two-tailed t-test).
H and I. A. phagocytophilum-infected MDR and drug-sensitive HL-60 cells at 24 h pi were collected (H) and A. phagocytophilum-infected HL-60 cells at day 1 pi were treated with U18666A (5 µM) for 24 h (I), stained with anti-NPC1 (green) and antibody 5C11 (red), and analyzed by fluorescence microscopy. The data shown are representative of at least three independent experiments. Each dotted line depicts the cell boundary. Bar, 5 µm. Ap, A. phagocytophilum.