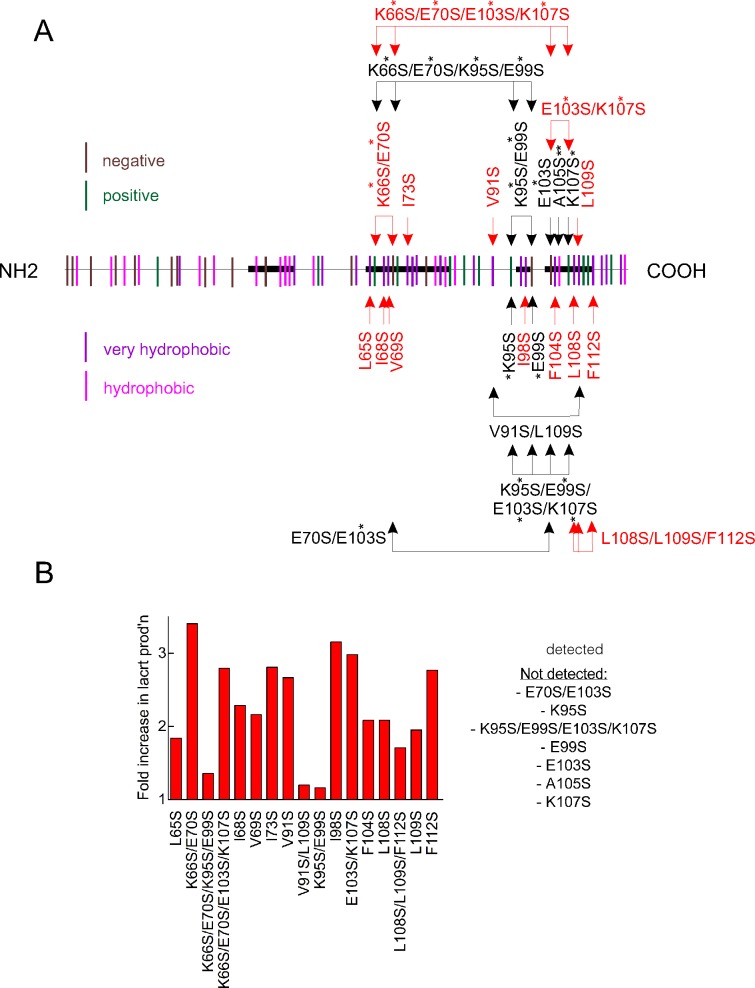
Figure 1.
Selective point mutagenesis of lacritin's hydrophobic residues, and a charged pair, substantially improves protein yield in E. coli. (A) Distribution of all hydrophobic (magenta) and very hydrophobic (purple) residues, as well as positively (green) and negatively (brown) charged residues along the length of lacritin. Shown is lacritin without the signal peptide. Point mutants under study are indicated. Double and single asterisks, respectively, indicate conservative and semiconservative substitutions. All other substitutions are radical. (B) Fold increase in purified mutant lacritin generated versus unaltered lacritin from 1 L productions runs of each that had been purified on chitin columns, released without tag with β-mercaptoethanol, concentrated by Amicon ultraspin filtration (EMD Millipore Corp.) on a 3 kDa molecular weight cutoff cartridge, dialyzed versus 5 L of PBS and then purified further on DEAE Sepharose. Purified lacritin was quantitated by the BCA protein assay.